| - |
Matter, Energy,
and Direct Current |
| - |
Alternating Current and Transformers |
| - |
Circuit Protection, Control, and Measurement |
| - |
Electrical Conductors, Wiring Techniques,
and Schematic Reading |
| - |
Generators and Motors |
| - |
Electronic Emission, Tubes, and Power Supplies |
| - |
Solid-State Devices and Power Supplies |
| - |
Amplifiers |
| - |
Wave-Generation and Wave-Shaping Circuits |
| - |
Wave Propagation, Transmission Lines, and
Antennas |
| - |
Microwave Principles |
| - |
Modulation Principles |
| - |
Introduction to Number Systems and Logic Circuits |
| - |
- Introduction to Microelectronics |
| - |
Principles of Synchros, Servos, and Gyros |
| - |
Introduction to Test Equipment |
| - |
Radio-Frequency Communications Principles |
| - |
Radar Principles |
| - |
The Technician's Handbook, Master Glossary |
| - |
Test Methods and Practices |
| - |
Introduction to Digital Computers |
| - |
Magnetic Recording |
| - |
Introduction to Fiber Optics |
| Note: Navy Electricity and Electronics Training
Series (NEETS) content is U.S. Navy property in the public domain. |
CHAPTER
1 | 2 | 3
LEARNING OBJECTIVES
Learning objectives are stated at the beginning each chapter. These learning
objectives serve as a preview the information you are expected to learn in the chapter.
The comprehensive check questions are based on the objectives. By successfully completing
the NRTC, you indicate that you have met the objectives and have learned the information.
The learning objectives are listed below.
Upon completing this chapter, you will be able to:
1. State the meanings and the relationship between matter, element, nucleus,
compound, molecule, mixture, atom, electron, proton, neutron, energy, valence, valence
shell, and ion.
2. State the meanings and the relationship between kinetic energy, potential
energy, photons, electron orbits, energy levels, and shells and subshells.
3. State, in terms valence, the differences between a conductor, an insulator,
and a semiconductor, and list some materials which make the best conductors and
insulators.
4. State the definition static electricity and explain how static electricity
is generated.
5. State the meanings retentivity, reluctance, permeability, ferromagnetism,
natural magnet, and artificial magnet as used to describe magnetic materials.
6. State the Weber and domain theories magnetism and list six characteristics
magnetic lines force (magnetic flux), including their relation to magnetic induction,
shielding, shape, and storage.
7. State, using the water analogy, how a difference potential (a voltage
or an electromotive force) can exist. Convert volts to microvolts, to millivolts,
and to kilovolts.
8. List six methods for producing a voltage (EMF) and state the operating
principles and the uses for each method.
9. State the meanings electron current, random drift, directed drift, and
ampere, and indicate the direction that an electric current flows.
10. State the relationship current to voltage and convert amperes to milliamperes
and microamperes.
11. State the definitions and the terms and symbols for resistance and
conductance, and how the temperature, contents, length and cross-sectional area
a conductor affect its resistance and conductance values.
12. List the physical and operating characteristics and the symbols, ratings,
and uses for various types resistors; use the color code to identify resistor values.
INTRODUCTION
The origin the modern technical and electronic Navy stretches back to the beginning
naval history, when the first navies were no more than small fleets wooden ships,
using wind-filled sails and manned oars. The need for technicians then was restricted
to a navigator and semiskilled seamen who could handle the sails.
As time passed, larger ships that carried more sail were built. These ships,
encouraging exploration and commerce, helped to establish world trade routes. Soon
strong navies were needed to guard these sea lanes. Countries established their
own navies to protect their citizens, commercial ships, and shipping lanes against
pirates and warring nations. With the addition mounted armament, gunners joined
the ship's company skilled or semiskilled technicians.
The advent the steam engine signaled the rise an energy source more practical
than either wind and sails or manpower. With this technological advancement, the
need for competent operators and technicians increased.
However, the big call for operators and technicians in the U.S. Navy came in
the early part the 20th century, when power sources, means communication, modes
detection, and armaments moved with amazing rapidity toward involved technical development.
Electric motors and generators by then had become the most widely used sources power.
Telephone systems were well established on board ship, and radio was being used
more and more to relay messages from ship to ship and from ship to shore. Listening
devices were employed to detect submarines. Complex optical systems were used to
aim large naval rifles. Mines and torpedoes became highly developed, effective weapons,
and airplanes joined the Navy team.
During the years after World War I, the Navy became more electricity and electronic
minded. It was recognized that a better system communications was needed aboard
each ship, and between the ships, planes, submarines, and shore installations; and
that weaponry advances were needed to keep pace with worldwide developments in that
field. This growing technology carried with it the awareness that an equally skilled
force technicians was needed for maintenance and service duties.
World War II proved that all the expense providing equipment for the fleet and
training personnel to handle that equipment paid great dividends. The U. S. Navy
had the modern equipment and highly trained personnel needed to defeat the powerful
fleets the enemy.
Today there is scarcely anyone on board a Navy ship who does not use electrical
or electronic equipment. This equipment is needed in systems electric lighting and
power, intercommunications, radio, radar, sonar, loran, remote metering, weapon
aiming, and certain types mines and torpedoes. The Navy needs trained operators
and technicians in this challenging field electronics and electricity. It is to
achieve this end that this module, and others like it, are published.
MATTER, ENERGY, and ELECTRICITY
If there are roots to western science, they no doubt lie under the rubble that
was once ancient Greece. With the exception the Greeks, ancient people had little
interest in the structure materials. They accepted a solid as being just that a
continuous, uninterrupted substance. One Greek school thought believed that if a
piece matter, such as copper, were subdivided, it could be done indefinitely and
still only that material would be found. Others reasoned that there must be a limit
to the number subdivisions that could be made and have the material still retain
its original characteristics. They held fast to the idea that there must be a
basic particle upon which all substances are built. Recent experiments have revealed
that there are, indeed, several basic particles, or building blocks within all substances.
The following paragraphs explain how substances are classified as elements and
compounds, and are made up molecules and atoms. This, then, will be a learning experience
about protons, electrons, valence, energy levels, and the physics electricity.
MATTER
Matter is defined as anything that occupies space and has weight; that is, the
weight and dimensions matter can be measured. Examples matter are air, water, automobiles,
clothing, and even our own bodies. Thus, we can say that matter may be found in
any one three states: SOLID, LIQUID, and GASEOUS.
ELEMENTS and COMPOUNDS
An ELEMENT is a substance which cannot be reduced to a simpler substance by chemical
means. Examples elements with which you are in everyday contact are iron, gold,
silver, copper, and oxygen. There are now over 100 known elements. All the different
substances we know about are composed one or more these elements.
When two or more elements are chemically combined, the resulting substance is
called a COMPOUND. a compound is a chemical combination elements which can be separated
by chemical but not by physical means. Examples common compounds are water which
consists hydrogen and oxygen, and table salt, which consists sodium and chlorine.
a MIXTURE, on the other hand, is a combination elements and compounds, not chemically
combined, that can be separated by physical means. Examples mixtures are air, which
is made up nitrogen, oxygen, carbon dioxide, and small amounts several rare gases,
and sea water, which consists chiefly salt and water.
Q1. What is matter, and in what three states is it found?
Q2. What is an element?
Q3. What is a compound?
Q4. What is the difference between a compound and a mixture?
MOLECULES
A MOLECULE is a chemical combination two or more atoms, (atoms are described
in the next paragraph). In a compound the molecule is the smallest particle that
has all the characteristics the compound.
Consider water, for example. Water is matter, since it occupies space and has
weight. Depending on the temperature, it may exist as a liquid (water), a solid
(ice), or a gas (steam). Regardless the temperature, it will still have the same
composition. If we start with a quantity water, divide this and pour out one half,
and continue this process a sufficient number times, we will eventually end up with
a quantity water which cannot be further divided without ceasing to be water. This
quantity is called a molecule water. If this molecule water divided, instead two
parts water, there will be one part oxygen and two parts hydrogen (H 2 O).
ATOMS
Molecules are made up smaller particles called ATOMS. An atom is the smallest
particle an element that retains the characteristics that element. The atoms one
element, however, differ from
the atoms all other elements. Since there are over 100 known elements, there
must be over 100 different atoms, or a different atom for each element. Just as
thousands words can be made by combining the proper letters the alphabet, so thousands
different materials can be made by chemically combining the proper atoms.
Any particle that is a chemical combination two or more atoms is called a molecule.
The oxygen molecule consists two atoms oxygen, and the hydrogen molecule consists
two atoms hydrogen. Sugar, on the other hand, is a compound composed atoms carbon,
hydrogen, and oxygen. These atoms are combined into sugar molecules. Since the sugar
molecules can be broken down by chemical means into smaller and simpler units, we
cannot have sugar atoms.
The atoms each element are made up electrons, protons, and, in most cases, neutrons,
which are collectively called subatomic particles. Furthermore, the electrons, protons,
and neutrons one element are identical to those any other element. The reason that
there are different kinds elements is that the number and the arrangement electrons
and protons within the atom are different for the different elements.
The electron is considered to be a small negative charge electricity. The proton
has a positive charge electricity equal and opposite to the charge the electron.
Scientists have measured the mass and size the electron and proton, and they know
how much charge each possesses. The electron and proton each have the same quantity
charge, although the mass the proton is approximately 1837 times that the electron.
In some atoms there exists a neutral particle called a neutron. The neutron has
a mass approximately equal to that a proton, but it has no electrical charge. According
to a popular theory, the electrons, protons, and neutrons the atoms are thought
to be arranged in a manner similar to a miniature solar system. The protons and
neutrons form a heavy nucleus with a positive charge, around which the very light
electrons revolve.
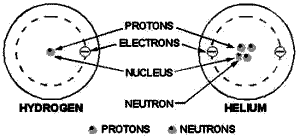
Figure 1-1. - Structures simple atoms.
Figure 1-1 shows one hydrogen and one helium atom. Each has a relatively simple
structure. The hydrogen atom has only one proton in the nucleus with one electron
rotating about it. The helium atom is a little more complex. It has a nucleus made
up two protons and two neutrons, with two electrons rotating about the nucleus.
Elements are classified numerically according to the complexity their atoms. The
atomic number an atom is determined by the number protons in its nucleus.
In a neutral state, an atom contains an equal number protons and electrons. Therefore,
an atom hydrogen - which contains one proton and one electron - has an atomic number
1; and helium, with
two protons and two electrons, has an atomic number 2. The complexity atomic
structure increases with the number protons and electrons.
Q5. What is a molecule?
Q6. What are the three types subatomic particles, and what are their charges?
ENERGY LEVELS
Since an electron in an atom has both mass and motion, it contains two types
energy. By virtue its motion the electron contains KINETIC ENERGY. Due to its position
it also contains POTENTIAL ENERGY. The total energy contained by an electron (kinetic
plus potential) is the factor which determines the radius the electron orbit. In
order for an electron to remain in this orbit, it must neither Gain nor LOSE energy.
It is well known that light is a form energy, but the physical form in which
this energy exists is not known.

Figure 1-2. - Excitation by a photon.
One accepted theory proposes the existence light as tiny packets energy called
PHOTONS. Photons can contain various quantities energy. The amount depends upon
the color the light involved. Should a photon sufficient energy collide with an
orbital electron, the electron will absorb the photon's energy, as shown in figure
1-2. The electron, which now has a greater than normal amount energy, will jump
to a new orbit farther from the nucleus. The first new orbit to which the electron
can jump has a radius four times as large as the radius the original orbit. Had
the electron received a greater amount energy, the next possible orbit to which
it could jump would have a radius nine times the original. Thus, each orbit may
be considered to represent one a large number energy levels that the electron may
attain. It must be emphasized that the electron cannot jump to just any orbit. The
electron will remain in its lowest orbit until a sufficient amount energy is available,
at which time the electron will accept the energy and jump to one a series permissible
orbits. An electron cannot exist in the space between energy levels. This indicates
that the electron will not accept a photon energy unless it contains enough energy
to elevate itself to one the higher energy levels. Heat energy and collisions with
other particles can also cause the electron to jump orbits.
Once the electron has been elevated to an energy level higher than the lowest
possible energy level, the atom is said to be in an excited state. The electron
will not remain in this excited condition for more than a fraction a second before
it will radiate the excess energy and return to a lower energy orbit. To illustrate
this principle, assume that a normal electron has just received a photon energy
sufficient to raise it from the first to the third energy level. In a short period
time the electron may jump back to the first level emitting a new photon identical
to the one it received.
A second alternative would be for the electron to return to the lower level in
two jumps; from the third to the second, and then from the second to the first.
In this case the electron would emit two photons, one for each jump. Each these
photons would have less energy than the original photon which excited the electron.
This principle is used in the fluorescent light where ultraviolet light photons,
which are not visible to the human eye, bombard a phosphor coating on the inside
a glass tube. The phosphor electrons, in returning to their normal orbits, emit
photons light that are visible. By using the proper chemicals for the phosphor coating,
any color light may be obtained, including white. This same principle is also used
in lighting up the screen a television picture tube.
The basic principles just developed apply equally well to the atoms more complex
elements. In atoms containing two or more electrons, the electrons interact with
each other and the exact path any one electron is very difficult to predict. However,
each electron lies in a specific energy band and the orbits will be considered as
an average the electron's position.
Q7. What is energy motion called?
Q8. How is invisible light changed to visible light in a fluorescent light?
SHELLS AND SUBSHELLS
The difference between the atoms, insofar as their chemical activity and stability
are concerned, is dependent upon the number and position the electrons included
within the atom. How are these electrons positioned within the atom? In general,
the electrons reside in groups orbits called shells. These shells are elliptically
shaped and are assumed to be located at fixed intervals. Thus, the shells are arranged
in steps that correspond to fixed energy levels. The shells, and the number electrons
required to fill them, may be predicted by the employment Pauli's exclusion principle.
Simply stated, this principle specifies that each shell will contain a maximum 2n2electrons,
where n corresponds to the shell number starting with the one closest to the nucleus.
By this principle, the second shell, for example, would contain 2(2) 2 or
8 electrons when full.

Figure 1-3. - Shell designation.

Figure 1-4. - Copper atom.
In addition to being numbered, the shells are also given letter designations,
as pictured in figure 1-3. Starting with the shell closest to the nucleus and progressing
outward, the shells are labeled K, L, M, N, O, P, and Q, respectively. The shells
are considered to be full, or complete, when they contain the following quantities
electrons: two in the K shell, eight in the L shell, 18 in the M shell, and so on,
in accordance with the exclusion principle. Each these shells is a major shell and
can be divided into subshells, which there are four, labeled s, p, d, and f. Like
the major shells, the subshells are also limited as to the number electrons which
they can contain. Thus, the "s" subshell is complete when it contains two electrons,
the "p" subshell when it contains 10, and the "f" subshell when it contains 14 electrons.
Inasmuch as the K shell can contain no more than two electrons, it must have
only one subshell, the s subshell. The M shell is composed three subshells: s, p,
and d. If the electrons in the s, p, and d subshells are added, their total is found
to be 18, the exact number required to fill the M shell. Notice the electron configuration
for copper illustrated in figure 1-4. The copper atom contains 29 electrons, which
completely fill the first three shells and subshells, leaving one electron in the
"s" subshell the N shell.
VALENCE
The number electrons in the outermost shell determines the valence an atom. For
this reason, the outer shell an atom is called the VALENCE SHELL; and the electrons
contained in this shell are called VALENCE ELECTRONS. The valence an atom determines
its ability to gain or lose an electron, which in turn determines the chemical and
electrical properties the atom. An atom that is lacking only one or two electrons
from its outer shell will easily gain electrons to complete its shell, but a large
amount energy is required to free any its electrons. An atom having a relatively
small number electrons in its outer shell in comparison to the number electrons
required to fill the shell will easily lose these valence electrons. The valence
shell always refers to the outermost shell.
Q9. What determines the valence an atom?
IONIZATION
When the atom loses electrons or gains electrons in this process electron exchange,
it is said to be IONIZED. For ionization to take place, there must be a transfer
energy which results in a change in the internal energy the atom. An atom having
more than its normal amount electrons acquires a negative charge, and is called
a Negative ION. The atom that gives up some its normal electrons is left with less
negative charges than positive charges and is called a Positive ION. Thus, ionization
is the process by which an atom loses or gains electrons.
Q1O. What is an ion?
Conductors, Semiconductors, and Insulators
In this study electricity and electronics, the association matter and electricity
is important. Since every electronic device is constructed parts made from ordinary
matter, the effects electricity on matter must be well understood. As a means accomplishing
this, all elements which matter is made may be placed into one three categories:
Conductors, Semiconductors, and Insulators, depending on their ability to conduct
an electric current. Conductors are elements which conduct electricity very readily,
Insulators have an extremely high resistance to the flow electricity. All matter
between these two extremes may be called Semiconductors.
The electron theory states that all matter is composed atoms and the atoms are
composed smaller particles called protons, electrons, and neutrons. The electrons
orbit the nucleus which contains the protons and neutrons. It is the valence electrons
that we are most concerned with in electricity. These are the electrons which are
easiest to break loose from their parent atom. Normally, conductors have three or
less valence electrons; insulators have five or more valence electrons; and semiconductors
usually have four valence electrons.
The electrical conductivity matter is dependent upon the atomic structure the
material from which the conductor is made. In any solid material, such as copper,
the atoms which make up the molecular structure are bound firmly together. At room
temperature, copper will contain a considerable amount heat energy. Since heat energy
is one method removing electrons from their orbits, copper will contain many free
electrons that can move from atom to atom. When not under the influence an external
force, these electrons move in a haphazard manner within the conductor. This movement
is equal in all directions so that electrons are not lost or gained by any part
the conductor. When controlled by an external force, the electrons move generally
in the same direction. The effect this movement is felt almost instantly from one
end the conductor to the other. This electron movement is called an ELECTRIC CURRENT.
Some metals are better conductors electricity than others. Silver, copper, gold,
and aluminum are materials with many free electrons and make good conductors. Silver
is the best conductor, followed by copper, gold, and aluminum. Copper is used more
ten than silver because cost. Aluminum is used where weight is a major consideration,
such as in high-tension power lines, with long spans between supports. Gold is used
where oxidation or corrosion is a consideration and a good conductivity is required.
The ability a conductor to handle current also depends upon its physical dimensions.
Conductors are usually found in the form wire, but may be in the form bars, tubes,
or sheets.
Nonconductors have few free electrons. These materials are called Insulators.
Some examples these materials are rubber, plastic, enamel, glass, dry wood, and
mica. Just as there is no perfect conductor, neither is there a perfect insulator.
Some materials are neither good conductors nor good insulators, since their electrical
characteristics fall between those conductors and insulators. These in-between materials
are classified as Semiconductors. Germanium and silicon are two common semiconductors used in solid-state devices.
Q11. What determines whether a substance is a conductor or an insulator?
ELECTROSTATICS
Electrostatics (electricity at rest) is a subject with which most persons entering
the field electricity and electronics are somewhat familiar. For example, the way
a person's hair stands on end after a vigorous rubbing is an effect electrostatics.
While pursuing the study electrostatics, you will gain a better understanding this
common occurrence. even greater significance, the study electrostatics will provide
you with the opportunity to gain important background knowledge and to develop concepts
which are essential to the understanding electricity and electronics.
Interest in the subject static electricity can be traced back to the Greeks.
Thales Miletus, a Greek philosopher and mathematician, discovered that when an amber
rod is rubbed with fur, the rod has the amazing characteristic attracting some very
light objects such as bits paper and shavings wood.
About 1600, William Gilbert, an English scientist, made a study other substances
which had been found to possess qualities attraction similar to amber. Among these
were glass, when rubbed with silk, and ebonite, when rubbed with fur. Gilbert classified
all the substances which possessed properties similar to those amber as electrics,
a word Greek origin meaning amber.
Because Gilbert's work with electrics, a substance such as amber or glass when
given a vigorous rubbing was recognized as being ELECTRIFIED, or CHARGED with electricity.
In the year 1733, Charles Dufay, a French scientist, made an important discovery
about electrification. He found that when a glass was rubbed with fur, both the
glass rod and the fur became electrified. This realization came when he systematically
placed the glass rod and the fur near other electrified substances and found that
certain substances which were attracted to the glass rod were repelled by the fur,
and vice versa. From experiments such as this, he concluded that there must be two
exactly opposite kinds electricity.
Benjamin Franklin, American statesman, inventor, and philosopher, is credited
with first using the terms Positive and Negative to describe the two opposite kinds
electricity. The charge produced on a glass rod when it is rubbed with silk, Franklin
labeled positive. He attached the term negative to the charge produced on the silk.
Those bodies which were not electrified or charged, he called NEUTRAL.
STATIC ELECTRICITY
In a natural, or neutral state, each atom in a body matter will have the proper
number electrons in orbit around it. Consequently, the whole body matter composed
the neutral atoms will also be electrically neutral. In this state, it is said to
have a "zero charge." Electrons will neither leave nor enter the neutrally charged
body should it come in contact with other neutral bodies. If, however, any number
electrons are removed from the atoms a body matter, there will remain more protons
than electrons and the whole body matter will become ELECTRICALLY Positive. Should
the positively charged body come in contact with another body having a normal charge,
or having a Negative (too many electrons) charge, an electric current will flow
between them. Electrons will leave the more negative body and enter the positive
body. This electron flow will continue until both bodies have equal charges. When
two bodies matter have unequal charges and are near one another, an electric force
is exerted between them because their unequal charges. However, since they are not
in contact, their charges cannot equalize. The existence such an electric force,
where current cannot flow, is referred to as static electricity. ("Static" in this
instance means "not moving.") It is also referred to as an electrostatic force.

Figure 1-5. - Producing static electricity by friction.
One the easiest ways to create a static charge is by friction. When two pieces
matter are rubbed together, electrons can be "wiped f" one material onto the other.
If the materials used are good conductors, it is quite difficult to obtain a detectable
charge on either, since equalizing currents can flow easily between the conducting
materials. These currents equalize the charges almost as fast as they are created.
a static charge is more easily created between nonconducting materials. When a hard
rubber rod is rubbed with fur, the rod will accumulate electrons given up by the
fur, as shown in figure 1-5. Since both materials are poor conductors, very little
equalizing current can flow, and an electrostatic charge builds up. When the charge
becomes great enough, current will flow regardless the poor conductivity the materials.
These currents will cause visible sparks and produce a crackling sound.
Q12. How is a negative charge created in a neutral body?
Q13. How are static charges created?
NATURE CHARGES
When in a natural, or neutral state, an atom has an equal number electrons and
protons. Because this balance, the net negative charge the electrons in orbit is
exactly balanced by the net positive charge the protons in the nucleus, making the
atom electrically neutral.
An atom becomes a positive ion whenever it loses an electron, and has an overall
positive charge. Conversely, whenever an atom acquires an extra electron, it becomes
a negative ion and has a negative charge.
Due to normal molecular activity, there are always ions present in any material.
If the number positive ions and negative ions is equal, the material is electrically
neutral. When the number positive ions exceeds the number negative ions, the material
is positively charged. The material is negatively charged whenever the negative
ions outnumber the positive ions.
Since ions are actually atoms without their normal number electrons, it is the
excess or the lack electrons in a substance that determines its charge. In most
solids, the transfer charges is by movement electrons rather than ions. The transfer
charges by ions will become more significant when we consider electrical activity
in liquids and gases. At this time, we will discuss electrical behavior in terms
electron movement.
Q14. What is the electrical charge an atom which contains 8 protons and
11 electrons?
CHARGED BODIES

Figure 1-6. - Reaction between charged bodies.
One the fundamental laws electricity is that LIKE CHARGES REPEL EACH OTHER and
UNLIKE CHARGES ATTRACT EACH OTHER. a positive charge and negative charge, being
unlike, tend to move toward each other. In the atom, the negative electrons are
drawn toward the positive protons in the nucleus. This attractive force is balanced
by the electron's centrifugal force caused by its rotation about the nucleus. As
a result, the electrons remain in orbit and are not drawn into the nucleus. Electrons
repel each other because their like negative charges, and protons repel each other
because their like positive charges.
The law charged bodies may be demonstrated by a simple experiment. Two pith (paper
pulp) balls are suspended near one another by threads, as shown in figure 1-6.
If a hard rubber rod is rubbed with fur to give it a negative charge and is then
held against the right- hand ball in part (A), the rod will give f a negative charge
to the ball. The right-hand ball will have a negative charge with respect to the
left-hand ball. When released, the two balls will be drawn together, as shown in
figure 1-6(A). They will touch and remain in contact until the left-hand ball gains
a portion the negative charge the right-hand ball, at which time they will swing
apart as shown in figure 1-6(C). If a positive or a negative charge is placed on
both balls (fig. 1-6(B)), the balls will repel each other.
Coulomb's Law Charges
The relationship between attracting or repelling charged bodies was first discovered
and written about by a French scientist named Charles A. Coulomb. Coulomb's Law
states that CHARGED BODIES ATTRACT OR REPEL EACH OTHER WITH a forCE THAT Is DirectLY
PROPORTIONAL to The Product THEIR INDIVIDUAL CHARGES, and Is INVERSELY PROPORTIONAL
to The SQUARE The DIsTANCE BETWEEN THEM.
The amount attracting or repelling force which acts between two electrically
charged bodies in free space depends on two things - (1) their charges and (2) the
distance between them.
Electric Fields
The space between and around charged bodies in which their influence is felt
is called an ELECTRIC FIELD forCE. It can exist in air, glass, paper, or a vacuum.
ELECTROSTATIC FIELDS and Dielectric FIELDS are other names used to refer to this
region force.
Fields force spread out in the space surrounding their point origin and, in general,
DIMINISH IN PROPORTION to the SQUARE of the DISTANCE FROM THEIR SOURCE.

Figure 1-7. - Electrostatic lines force.
The field about a charged body is generally represented by lines which are referred
to as ELECTROSTATIC Lines FORCE. These lines are imaginary and are used merely to
represent the direction and strength the field. To avoid confusion, the lines force
exerted by a positive charge are always shown leaving the charge, and for a negative
charge they are shown entering. Figure 1-7 illustrates the use lines to represent
the field about charged bodies.
Figure 1-7(A) represents the repulsion like-charged bodies and their associated
fields. Part (B) represents the attraction unlike-charged bodies and their associated
fields.
Q15. What is the relationship between charged bodies?
Q16. What is an electrostatic field?
Q17. In what direction are electrostatic lines force drawn?
MAGNETISM
In order to properly understand the principles electricity, it is necessary to
study magnetism and the effects magnetism on electrical equipment. Magnetism and
electricity are so closely related that the study either subject would be incomplete
without at least a basic knowledge the other.
Much today's modern electrical and electronic equipment could not function without
magnetism. Modern computers, tape recorders, and video reproduction equipment use
magnetized tape. High-fidelity speakers use magnets to convert amplifier outputs
into audible sound. Electrical motors use magnets to convert electrical energy into
mechanical motion; generators use magnets to convert mechanical motion into electrical
energy.
Q18. What are some examples electrical equipment which use magnetism?
MAGNETIC MATERIALS
Magnetism is generally defined as that property a material which enables it to
attract pieces iron. a material possessing this property is known as a MAGNET. The
word originated with the ancient Greeks, who found stones possessing this characteristic.
Materials that are attracted by a magnet, such as iron, steel, nickel, and cobalt,
have the ability to become magnetized. These are called magnetic materials.
Materials, such as paper, wood, glass, or tin, which are not attracted by magnets,
are considered nonmagnetic. Nonmagnetic materials are not able to become magnetized.
Q19. What are magnetic materials?
FERROMAGNETIC MATERIALS
The most important group materials connected with electricity and electronics
are the ferromagnetic materials. Ferromagnetic materials are those which are relatively
easy to magnetize, such as iron, steel, cobalt, and the alloys Alnico and Permalloy.
(An alloy is made from combining two or more elements, one which must be a metal).
These new alloys can be very strongly magnetized, and are capable obtaining a magnetic
strength great enough to lift 500 times their own weight.
Natural Magnets
Magnetic stones such as those found by the ancient Greeks are considered to be
NATURAL MAGNETS. These stones had the ability to attract small pieces iron in a
manner similar to the magnets which are common today. However, the magnetic properties
attributed to the stones were products nature and not the result the efforts man.
The Greeks called these substances magnetite.
The Chinese are said to have been aware some the effects magnetism as early as
2600 B.C. They observed that stones similar to magnetite, when freely suspended,
had a tendency to assume a nearly north and south direction. Because the directional
quality these stones, they were later referred to as lodestones or leading stones.
Natural magnets, which presently can be found in the United States, Norway, and
Sweden, no longer have any practical use, for it is now possible to easily produce
more powerful magnets.
Q20. What characteristics do all ferromagnetic materials have in common?
ARTIFICIAL MAGNETS
Magnets produced from magnetic materials are called ARTIFICIAL MAGNETS. They
can be made in a variety shapes and sizes and are used extensively in electrical
apparatus. Artificial magnets are generally made from special iron or steel alloys
which are usually magnetized electrically. The material to be magnetized is inserted
into a coil insulated wire and a heavy flow electrons is passed through the wire.
Magnets can also be produced by stroking a magnetic material with magnetite or with
another artificial magnet. The forces causing magnetization are represented by magnetic
lines force, very similar in nature to electrostatic lines force.
Artificial magnets are usually classified as PERMANENT or TEMPORARY, depending
on their ability to retain their magnetic properties after the magnetizing force
has been removed. Magnets made from substances, such as hardened steel and certain
alloys which retain a great deal their magnetism, are called PERMANENT MAGNETS.
These materials are relatively difficult to magnetize because the opposition refered
to the magnetic lines force as the lines force try to distribute themselves throughout
the material. The opposition that a material refers to the magnetic lines force
is called RELUCTANCE. All permanent magnets are produced from materials having a
high reluctance.
A material with a low reluctance, such as st iron or annealed silicon steel,
is relatively easy to magnetize but will retain only a small part its magnetism
once the magnetizing force is removed. Materials this type that easily lose most
their magnetic strength are called TEMPORARY MAGNETS. The amount magnetism which
remains in a temporary magnet is referred to as its RESIDUAL MAGNETISM.
The ability a material to retain an amount residual magnetism is called the RETENTIVITY
the material.
The difference between a permanent and a temporary magnet has been indicated
in terms RELUCTANCE, a permanent magnet having a high reluctance and a temporary
magnet having a low reluctance. Magnets are also described in terms the PERMEABILITY
their materials, or the ease with which magnetic lines force distribute themselves
throughout the material. a permanent magnet, which is produced from a material with
a high reluctance, has a low permeability. a temporary magnet, produced from a material
with a low reluctance, would have a high permeability.
Q21. What type magnetic material should be used to make a temporary magnet?
Q22. What is retentivity?
MAGNETIC POLES
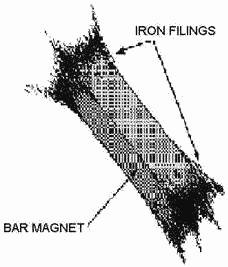
Figure 1-8. - Iron filings cling to the poles of a magnet.

Figure 1-9. - a bar magnet acts as a compass.

Figure 1-10. - The earth is a magnet.

Figure 1-11. - Weber's molecular theory magnetism.
The magnetic force surrounding a magnet is not uniform. There exists a great
concentration force at each end the magnet and a very weak force at the center.
Pro this fact can be obtained by dipping a magnet into iron filings (fig. 1-8).
It is found that many filings will cling to the ends the magnet while very few adhere
to the center. The two ends, which are the regions concentrated lines force, are
called the POLES the magnet. Magnets have two magnetic poles and both poles have
equal magnetic strength.
LAW of MAGNETIC POLES
If a bar magnet is suspended freely on a string, as shown in figure 1-9, it will
align itself in a north and south direction. When this experiment is repeated, it
is found that the same pole the magnet will always swing toward the north magnetic
pole the earth. Therefore, it is called the north-seeking pole or simply the NORTH
POLE. The other pole the magnet is the south-seeking pole or the SOUTH POLE.
A practical use the directional characteristic the magnet is the compass, a device
in which a freely rotating magnetized needle indicator points toward the North Pole.
The realization that the poles a suspended magnet always move to a definite position
gives an indication that the opposite poles a magnet have opposite magnetic polarity.
The law previously stated regarding the attraction and repulsion charged bodies
may also be applied to magnetism if the pole is considered as a charge. The north
pole a magnet will always be attracted to the south pole another magnet and will
show a repulsion to a north pole. The law for magnetic poles is:
Like poles repel, unlike poles attract.
Q23. How does the law magnetic poles relate to the law electric charges?
The Earth's Magnetic Poles
The fact that a compass needle always aligns itself in a particular direction,
regardless its location on earth, indicates that the earth is a huge natural magnet.
The distribution the magnetic force about the earth is the same as that which might
be produced by a giant bar magnet running through the center the earth (fig. 1-10).
The magnetic axis the earth is located about 15° from its geographical axis
thereby locating the magnetic poles some distance from the geographical poles. The
ability the north pole the compass needle to point toward the north geographical
pole is due to the presence the magnetic pole nearby. This magnetic pole is named
the magnetic North Pole. However, in actuality, it must have the polarity a south
magnetic pole since it attracts the north pole a compass needle. The reason for
this conflict in terminology can be traced to the early users the compass. Knowing
little about magnetic effects, they called the end the compass needle that pointed
towards the north geographical pole, the north pole a compass. With our present
knowledge magnetism, we know the north pole a compass needle (a small bar magnet)
can be attracted only by an unlike magnetic pole, that is, a pole south magnetic
polarity.
Q24. a compass is located at the geographical North Pole. In which direction
would its needle pointy
THEORIES MAGNETISM WEBER'S THEORY
A popular theory magnetism considers the molecular alignment the material. This
is known as Weber's theory. This theory assumes that all magnetic substances are
composed tiny molecular magnets. Any unmagnetized material has the magnetic forces
its molecular magnets neutralized by adjacent molecular magnets, thereby eliminating
any magnetic effect. a magnetized material will have most its molecular magnets
lined up so that the north pole each molecule points in one direction, and the south
pole faces the opposite direction. a material with its molecules thus aligned will
then have one effective north pole, and one effective south pole. An illustration
Weber's Theory is shown in figure 1-11, where a steel bar is magnetized by stroking.
When a steel bar is stroked several times in the same direction by a magnet, the
magnetic force from the north pole the magnet causes the molecules to align themselves.
Q25. Using Weber's molecular theory magnetism, describe the polarity the
magnetic poles produced by stroking a magnetic material from right to left with
the south pole a magnet.
DOMAIN THEORY
A more modern theory magnetism is based on the electron spin principle. From
the study atomic structure it is known that all matter is composed vast quantities
atoms, each atom containing one or more orbital electrons. The electrons are considered
to orbit in various shells and subshells depending upon their distance from the
nucleus. The structure the atom has previously been compared to the solar system,
wherein the electrons orbiting the nucleus correspond to the planets orbiting the
sun. Along with its orbital motion about the sun, each planet also revolves on its
axis. It is believed that the electron also revolves on its axis as it orbits the
nucleus an atom.
It has been experimentally proven that an electron has a magnetic field about
it along with an electric field. The effectiveness the magnetic field an atom is
determined by the number electrons spinning in each direction. If an atom has equal
numbers electrons spinning in opposite directions, the magnetic fields surrounding
the electrons cancel one another, and the atom is unmagnetized. However, if more
electrons spin in one direction than another, the atom is magnetized. An atom with
an atomic number 26, such as iron, has 26 protons in the nucleus and 26 revolving
electrons orbiting its nucleus.

Figure 1-12. - Iron atom.
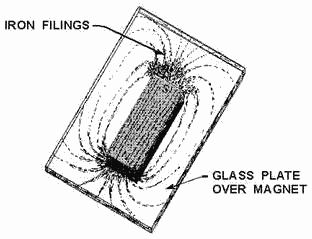
Figure 1-13. - Pattern formed by iron filings.

Figure 1-14. - Bar magnet showing lines of force.
If 13 electrons are spinning in a clockwise direction and 13 electrons are spinning
in a counterclockwise direction, the opposing magnetic fields will be neutralized.
When more than 13 electrons spin in either direction, the atom is magnetized. An
example a magnetized atom iron is shown in figure 1-12.
Q26. What is the difference between the domain theory and Weber‘s theory
magnetism?
MAGNETIC FIELDS
The space surrounding a magnet where magnetic forces act is known as the magnetic
field.
A pattern this directional force can be obtained by performing an experiment
with iron filings. a piece glass is placed over a bar magnet and the iron filings
are then sprinkled on the surface the glass. The magnetizing force the magnet will
be felt through the glass and each iron filing becomes a temporary magnet. If the
glass is now tapped gently, the iron particles will align themselves with the magnetic
field surrounding the magnet just as the compass needle did previously. The filings
form a definite pattern, which is a visible representation the forces comprising
the magnetic field. Examination the arrangements iron filings in figure 1-13 will
indicate that the magnetic field is very strong at the poles and weakens as the
distance from the poles increases. It is also apparent that the magnetic field extends
from one pole to the other, constituting a loop about the magnet.
Q27. Refer to figure 1-13. For what purpose would you sprinkle iron filings
on the glass plate?
Q28. Refer to figure 1-13. What pattern would be formed if sawdust was
sprinkled on the glass instead iron filings?
Lines of FORCE
To further describe and work with magnet phenomena, lines are used to represent
the force existing in the area surrounding a magnet (refer to fig. 1-14). These
lines, called Magnetic Lines FORCE, do not actually exist but are imaginary lines used to illustrate and describe the pattern the magnetic field. The magnetic lines
force are assumed to emanate from the north pole a magnet, pass through surrounding
space, and enter the south pole. The lines force then travel inside the magnet from
the south pole to the north pole, thus completing a closed loop.
|





















