| - |
Matter, Energy,
and Direct Current |
| - |
Alternating Current and Transformers |
| - |
Circuit Protection, Control, and Measurement |
| - |
Electrical Conductors, Wiring Techniques,
and Schematic Reading |
| - |
Generators and Motors |
| - |
Electronic Emission, Tubes, and Power Supplies |
| - |
Solid-State Devices and Power Supplies |
| - |
Amplifiers |
| - |
Wave-Generation and Wave-Shaping Circuits |
| - |
Wave Propagation, Transmission Lines, and
Antennas |
| - |
Microwave Principles |
| - |
Modulation Principles |
| - |
Introduction to Number Systems and Logic Circuits |
| - |
- Introduction to Microelectronics |
| - |
Principles of Synchros, Servos, and Gyros |
| - |
Introduction to Test Equipment |
| - |
Radio-Frequency Communications Principles |
| - |
Radar Principles |
| - |
The Technician's Handbook, Master Glossary |
| - |
Test Methods and Practices |
| - |
Introduction to Digital Computers |
| - |
Magnetic Recording |
| - |
Introduction to Fiber Optics |
| Note: Navy Electricity and Electronics Training
Series (NEETS) content is U.S. Navy property in the public domain. |
Chapter 1
Matter, Energy, and Electricity
Learning Objectives
Learning objectives are stated at the beginning each chapter. These learning
objectives serve as a preview the information you are expected to learn in the chapter.
The comprehensive check questions are based on the objectives. By successfully completing
the NRTC, you indicate that you have met the objectives and have learned the information.
The learning objectives are listed below.
Upon completing this chapter, you will be able to:
1. State the meanings and the relationship between matter, element, nucleus,
compound, molecule, mixture, atom, electron, proton, neutron, energy, valence, valence
shell, and ion.
2. State the meanings and the relationship between kinetic energy, potential
energy, photons, electron orbits, energy levels, and shells and subshells.
3. State, in terms valence, the differences between a conductor, an insulator,
and a semiconductor, and list some materials which make the best conductors and
insulators.
4. State the definition static electricity and explain how static electricity
is generated.
5. State the meanings retentivity, reluctance, permeability, ferromagnetism,
natural magnet, and artificial magnet as used to describe magnetic materials.
6. State the Weber and domain theories magnetism and list six characteristics
magnetic lines force (magnetic flux), including their relation to magnetic induction,
shielding, shape, and
storage.
7. State, using the water analogy, how a difference potential (a voltage
or an electromotive force) can exist. Convert volts to microvolts, to millivolts,
and to kilovolts.
8. List six methods for producing a voltage (EMF) and state the operating
principles and the uses for each method.
9. State the meanings electron current, random drift, directed drift, and
ampere, and indicate the direction that an electric current flows.
10. State the relationship current to voltage and convert amperes to milliamperes
and microamperes.
11. State the definitions and the terms and symbols for resistance and
conductance, and how the temperature, contents, length and cross-sectional area
a conductor affect its resistance and conductance values.
12. List the physical and operating characteristics and the symbols, ratings,
and uses for various types resistors; use the color code to identify resistor values.
Introduction
The origin the modern technical and electronic Navy stretches back to the beginning
naval history, when the first navies were no more than small fleets wooden ships,
using wind-filled sails and manned oars. The need for technicians then was restricted
to a navigator and semiskilled seamen who could handle the sails.
As time passed, larger ships that carried more sail were built. These ships,
encouraging exploration and commerce, helped to establish world trade routes. Soon
strong navies were needed to guard these sea lanes. Countries established their
own navies to protect their citizens, commercial ships, and shipping lanes against
pirates and warring nations. With the addition mounted armament, gunners joined
the ship's company skilled or semiskilled technicians.
The advent the steam engine signaled the rise an energy source more practical
than either wind and sails or manpower. With this technological advancement, the
need for competent operators and technicians increased.
However, the big call for operators and technicians in the U.S. Navy came in
the early part the 20th century, when power sources, means communication, modes
detection, and armaments moved with amazing rapidity toward involved technical development.
Electric motors and generators by then had become the most widely used sources power.
Telephone systems were well established on board ship, and radio was being used
more and more to relay messages from ship to ship and from ship to shore. Listening
devices were employed to detect submarines. Complex optical systems were used to
aim large naval rifles. Mines and torpedoes became highly developed, effective weapons,
and airplanes joined the Navy team.
During the years after World War I, the Navy became more electricity and electronic
minded. It was recognized that a better system communications was needed aboard
each ship, and between the ships, planes, submarines, and shore installations; and
that weaponry advances were needed to keep pace with worldwide developments in that
field. This growing technology carried with it the awareness that an equally skilled
force technicians was needed for maintenance and service duties.
World War II proved that all the expense providing equipment for the fleet and
training personnel to handle that equipment paid great dividends. The U. S. Navy
had the modern equipment and highly trained personnel needed to defeat the powerful
fleets the enemy.
Today there is scarcely anyone on board a Navy ship who does not use electrical
or electronic equipment. This equipment is needed in systems electric lighting and
power, intercommunications, radio, radar, sonar, loran, remote metering, weapon
aiming, and certain types mines and torpedoes. The Navy needs trained operators
and technicians in this challenging field electronics and electricity. It is to
achieve this end that this module, and others like it, are published.
MATTER, ENERGY, and ELECTRICITY
If there are roots to western science, they no doubt lie under the rubble that
was once ancient Greece. With the exception the Greeks, ancient people had little
interest in the structure materials. They accepted a solid as being just that a
continuous, uninterrupted substance. One Greek school thought believed that if a
piece matter, such as copper, were subdivided, it could be done indefinitely and
still only that material would be found. Others reasoned that there must be a limit
to the number subdivisions that could be made and have the material still retain
its original characteristics. They held
fast to the idea that there must be a basic particle upon which all substances
are built. Recent experiments have revealed that there are, indeed, several basic
particles, or building blocks within all substances.
The following paragraphs explain how substances are classified as elements and
compounds, and are made up molecules and atoms. This, then, will be a learning experience
about protons, electrons, valence, energy levels, and the physics electricity.
MATTER
Matter is defined as anything that occupies space and has weight; that is, the
weight and dimensions matter can be measured. Examples matter are air, water, automobiles,
clothing, and even our own bodies. Thus, we can say that matter may be found in
any one three states: SOLID, LIQUID, and GASEOUS.
ELEMENTS and COMPOUNDS
An ELEMENT is a substance which cannot be reduced to a simpler substance by chemical
means. Examples elements with which you are in everyday contact are iron, gold,
silver, copper, and oxygen. There are now over 100 known elements. All the different
substances we know about are composed one or more these elements.
When two or more elements are chemically combined, the resulting substance is
called a COMPOUND. a compound is a chemical combination elements which can be separated
by chemical but not by physical means. Examples common compounds are water which
consists hydrogen and oxygen, and table salt, which consists sodium and chlorine.
a MIXTURE, on the other hand, is a combination elements and compounds, not chemically
combined, that can be separated by physical means. Examples mixtures are air, which
is made up nitrogen, oxygen, carbon dioxide, and small amounts several rare gases,
and sea water, which consists chiefly salt and water.
Q1. What is matter, and in what three states is it found?
Q2. What is an element?
Q3. What is a compound?
Q4. What is the difference between a compound and a mixture?
MOLECULES
A MOLECULE is a chemical combination two or more atoms, (atoms are described
in the next paragraph). In a compound the molecule is the smallest particle that
has all the characteristics the compound.
Consider water, for example. Water is matter, since it occupies space and has
weight. Depending on the temperature, it may exist as a liquid (water), a solid
(ice), or a gas (steam). Regardless the temperature, it will still have the same
composition. If we start with a quantity water, divide this and pour out one half,
and continue this process a sufficient number times, we will eventually end up with
a quantity water which cannot be further divided without ceasing to be water. This
quantity is called a molecule water. If this molecule water divided, instead two
parts water, there will be one part oxygen and two parts hydrogen (H 2 O).
ATOMS
Molecules are made up smaller particles called ATOMS. An atom is the smallest
particle an element that retains the characteristics that element. The atoms one
element, however, differ from
the atoms all other elements. Since there are over 100 known elements, there
must be over 100 different atoms, or a different atom for each element. Just as
thousands words can be made by combining the proper letters the alphabet, so thousands
different materials can be made by chemically combining the proper atoms.
Any particle that is a chemical combination two or more atoms is called a molecule.
The oxygen molecule consists two atoms oxygen, and the hydrogen molecule consists
two atoms hydrogen. Sugar, on the other hand, is a compound composed atoms carbon,
hydrogen, and oxygen. These atoms are combined into sugar molecules. Since the sugar
molecules can be broken down by chemical means into smaller and simpler units, we
cannot have sugar atoms.
The atoms each element are made up electrons, protons, and, in most cases, neutrons,
which are collectively called subatomic particles. Furthermore, the electrons, protons,
and neutrons one element are identical to those any other element. The reason that
there are different kinds elements is that the number and the arrangement electrons
and protons within the atom are different for the different elements.
The electron is considered to be a small negative charge electricity. The proton
has a positive charge electricity equal and opposite to the charge the electron.
Scientists have measured the mass and size the electron and proton, and they know
how much charge each possesses. The electron and proton each have the same quantity
charge, although the mass the proton is approximately 1837 times that the electron.
In some atoms there exists a neutral particle called a neutron. The neutron has
a mass approximately equal to that a proton, but it has no electrical charge. According
to a popular theory, the electrons, protons, and neutrons the atoms are thought
to be arranged in a manner similar to a miniature solar system. The protons and
neutrons form a heavy nucleus with a positive charge, around which the very light
electrons revolve.
Figure 1-1 shows one hydrogen and one helium atom. Each has a relatively simple
structure. The hydrogen atom has only one proton in the nucleus with one electron
rotating about it. The helium atom is a little more complex. It has a nucleus made
up two protons and two neutrons, with two electrons rotating about the nucleus.
Elements are classified numerically according to the complexity their atoms. The
atomic number an atom is determined by the number protons in its nucleus.
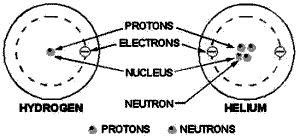
Figure 1-1. - Structures simple atoms.
In a neutral state, an atom contains an equal number protons and electrons. Therefore,
an atom hydrogen - which contains one proton and one electron - has an atomic number
1; and helium, with
two protons and two electrons, has an atomic number 2. The complexity atomic
structure increases with the number protons and electrons.
Q5. What is a molecule?
Q6. What are the three types subatomic particles, and what are their charges?
Energy Levels
Since an electron in an atom has both mass and motion, it contains two types
energy. By virtue its motion the electron contains KINETIC ENERGY. Due to its position
it also contains POTENTIAL ENERGY. The total energy contained by an electron (kinetic
plus potential) is the factor which determines the radius the electron orbit. In
order for an electron to remain in this orbit, it must neither Gain nor LOSE energy.
It is well known that light is a form energy, but the physical form in which
this energy exists is not known.
One accepted theory proposes the existence light as tiny packets energy called
PHOTONS. Photons can contain various quantities energy. The amount depends upon
the color the light involved. Should a photon sufficient energy collide with an
orbital electron, the electron will absorb the photon's energy, as shown in figure
1-2. The electron, which now has a greater than normal amount energy, will jump
to a new orbit farther from the nucleus. The first new orbit to which the electron
can jump has a radius four times as large as the radius the original orbit. Had
the electron received a greater amount energy, the next possible orbit to which
it could jump would have a radius nine times the original. Thus, each orbit may
be considered to represent one a large number energy levels that the electron may
attain. It must be emphasized that the electron cannot jump to just any orbit. The
electron will remain in its lowest orbit until a sufficient amount energy is available,
at which time the electron will accept the energy and jump to one a series permissible
orbits. An electron cannot exist in the space between energy levels. This indicates
that the electron will not accept a photon energy unless it contains enough energy
to elevate itself to one the higher energy levels. Heat energy and collisions with
other particles can also cause the electron to jump orbits.

Figure 1-2. - Excitation by a photon.
Once the electron has been elevated to an energy level higher than the lowest
possible energy level, the atom is said to be in an excited state. The electron
will not remain in this excited condition for more than a fraction a second before
it will radiate the excess energy and return to a lower energy orbit. To illustrate
this principle, assume that a normal electron has just received a photon energy
sufficient to raise it from the first to the third energy level. In a short period
time the electron may jump back to the first level emitting a new photon identical
to the one it received.
A second alternative would be for the electron to return to the lower level in
two jumps; from the third to the second, and then from the second to the first.
In this case the electron would emit two photons, one for each jump. Each these
photons would have less energy than the original photon which excited the electron.
This principle is used in the fluorescent light where ultraviolet light photons,
which are not visible to the human eye, bombard a phosphor coating on the inside
a glass tube. The phosphor electrons, in returning to their normal orbits, emit
photons light that are visible. By using the proper chemicals for the phosphor coating,
any color light may be obtained, including white. This same principle is also used
in lighting up the screen a television picture tube.
The basic principles just developed apply equally well to the atoms more complex
elements. In atoms containing two or more electrons, the electrons interact with
each other and the exact path any one electron is very difficult to predict. However,
each electron lies in a specific energy band and the orbits will be considered as
an average the electron's position.
Q7. What is energy motion called?
Q8. How is invisible light changed to visible light in a fluorescent light?
Shells and Subshells
The difference between the atoms, insofar as their chemical activity and stability
are concerned, is dependent upon the number and position the electrons included
within the atom. How are these electrons positioned within the atom? In general,
the electrons reside in groups orbits called shells. These shells are elliptically
shaped and are assumed to be located at fixed intervals. Thus, the shells are arranged
in steps that correspond to fixed energy levels. The shells, and the number electrons
required to fill them, may be predicted by the employment Pauli's exclusion principle.
Simply stated, this principle specifies that each shell will contain a maximum 2n2electrons,
where n corresponds to the shell number starting with the one closest to the nucleus.
By this principle, the second shell, for example, would contain 2(2) 2 or
8 electrons when full.
In addition to being numbered, the shells are also given letter designations,
as pictured in figure 1-3. Starting with the shell closest to the nucleus and progressing
outward, the shells are labeled K, L, M, N, O, P, and Q, respectively. The shells
are considered to be full, or complete, when they contain the following quantities
electrons: two in the K shell, eight in the L shell, 18 in the M shell, and so on,
in accordance with the exclusion principle. Each these shells is a major shell and
can be divided into subshells, which there are four, labeled s, p, d, and f. Like
the major shells, the subshells are also limited as to the number electrons which
they can contain. Thus, the "s" subshell is complete when it contains two electrons,
the "p" subshell when it contains 10, and the "f" subshell when it contains 14 electrons.

Figure 1-3. - Shell designation.
Inasmuch as the K shell can contain no more than two electrons, it must have
only one subshell, the s subshell. The M shell is composed three subshells: s, p,
and d. If the electrons in the s, p, and d subshells are added, their total is found
to be 18, the exact number required to fill the M shell. Notice the electron configuration
for copper illustrated in figure 1-4. The copper atom contains 29 electrons, which
completely fill the first three shells and subshells, leaving one electron in the
"s" subshell the N shell.

Figure 1-4. - Copper atom.
Valence
The number electrons in the outermost shell determines the valence an atom. For
this reason, the outer shell an atom is called the VALENCE SHELL; and the electrons
contained in this shell are called VALENCE ELECTRONS. The valence an atom determines
its ability to gain or lose an electron, which in turn determines the chemical and
electrical properties the atom. An atom that is
lacking only one or two electrons from its outer shell will easily gain electrons
to complete its shell, but a large amount energy is required to free any its electrons.
An atom having a relatively small number electrons in its outer shell in comparison
to the number electrons required to fill the shell will easily lose these valence
electrons. The valence shell always refers to the outermost shell.
Q9. What determines the valence an atom?
Ionization
When the atom loses electrons or gains electrons in this process electron exchange,
it is said to be IONIZED. For ionization to take place, there must be a transfer
energy which results in a change in the internal energy the atom. An atom having
more than its normal amount electrons acquires a negative charge, and is called
a Negative ION. The atom that gives up some its normal electrons is left with less
negative charges than positive charges and is called a Positive ION. Thus, ionization
is the process by which an atom loses or gains electrons.
Q1O. What is an ion?
Conductors, Semiconductors, and Insulators
In this study electricity and electronics, the association matter and electricity
is important. Since every electronic device is constructed parts made from ordinary
matter, the effects electricity on matter must be well understood. As a means accomplishing
this, all elements which matter is made may be placed into one three categories:
Conductors, Semiconductors, and Insulators, depending on their ability to conduct
an electric current. Conductors are elements which conduct electricity very readily,
Insulators have an extremely high resistance to the flow electricity. All matter
between these two extremes may be called Semiconductors.
The electron theory states that all matter is composed atoms and the atoms are
composed smaller particles called protons, electrons, and neutrons. The electrons
orbit the nucleus which contains the protons and neutrons. It is the valence electrons
that we are most concerned with in electricity. These are the electrons which are
easiest to break loose from their parent atom. Normally, conductors have three or
less valence electrons; insulators have five or more valence electrons; and semiconductors
usually have four valence electrons.
The electrical conductivity matter is dependent upon the atomic structure the
material from which the conductor is made. In any solid material, such as copper,
the atoms which make up the molecular structure are bound firmly together. At room
temperature, copper will contain a considerable amount heat energy. Since heat energy
is one method removing electrons from their orbits, copper will contain many free
electrons that can move from atom to atom. When not under the influence an external
force, these electrons move in a haphazard manner within the conductor. This movement
is equal in all directions so that electrons are not lost or gained by any part
the conductor. When controlled by an external force, the electrons move generally
in the same direction. The effect this movement is felt almost instantly from one
end the conductor to the other. This electron movement is called an ELECTRIC CURRENT.
Some metals are better conductors electricity than others. Silver, copper, gold,
and aluminum are materials with many free electrons and make good conductors. Silver
is the best conductor, followed by copper, gold, and aluminum. Copper is used more
ten than silver because cost. Aluminum is used where weight is a major consideration,
such as in high-tension power lines, with long spans between supports. Gold is used
where oxidation or corrosion is a consideration and a good conductivity is
required. The ability a conductor to handle current also depends upon its physical
dimensions. Conductors are usually found in the form wire, but may be in the form
bars, tubes, or sheets.
Nonconductors have few free electrons. These materials are called Insulators.
Some examples these materials are rubber, plastic, enamel, glass, dry wood, and
mica. Just as there is no perfect conductor, neither is there a perfect insulator.
Some materials are neither good conductors nor good insulators, since their electrical
characteristics fall between those conductors and insulators. These in-between materials
are classified as Semiconductors. Germanium and silicon are two common semiconductors used in solid-state devices.
Q11. What determines whether a substance is a conductor or an insulator?
ELECTROSTATICS
Electrostatics (electricity at rest) is a subject with which most persons entering
the field electricity and electronics are somewhat familiar. For example, the way
a person's hair stands on end after a vigorous rubbing is an effect electrostatics.
While pursuing the study electrostatics, you will gain a better understanding this
common occurrence. even greater significance, the study electrostatics will provide
you with the opportunity to gain important background knowledge and to develop concepts
which are essential to the understanding electricity and electronics.
Interest in the subject static electricity can be traced back to the Greeks.
Thales Miletus, a Greek philosopher and mathematician, discovered that when an amber
rod is rubbed with fur, the rod has the amazing characteristic attracting some very
light objects such as bits paper and shavings wood.
About 1600, William Gilbert, an English scientist, made a study other substances
which had been found to possess qualities attraction similar to amber. Among these
were glass, when rubbed with silk, and ebonite, when rubbed with fur. Gilbert classified
all the substances which possessed properties similar to those amber as electrics,
a word Greek origin meaning amber.
Because Gilbert's work with electrics, a substance such as amber or glass when
given a vigorous rubbing was recognized as being ELECTRIFIED, or CHARGED with electricity.
In the year 1733, Charles Dufay, a French scientist, made an important discovery
about electrification. He found that when a glass was rubbed with fur, both the
glass rod and the fur became electrified. This realization came when he systematically
placed the glass rod and the fur near other electrified substances and found that
certain substances which were attracted to the glass rod were repelled by the fur,
and vice versa. From experiments such as this, he concluded that there must be two
exactly opposite kinds electricity.
Benjamin Franklin, American statesman, inventor, and philosopher, is credited
with first using the terms Positive and Negative to describe the two opposite kinds
electricity. The charge produced on a glass rod when it is rubbed with silk, Franklin
labeled positive. He attached the term negative to the charge produced on the silk.
Those bodies which were not electrified or charged, he called NEUTRAL.
STATIC ELECTRICITY
In a natural, or neutral state, each atom in a body matter will have the proper
number electrons in orbit around it. Consequently, the whole body matter composed
the neutral atoms will also be
electrically neutral. In this state, it is said to have a "zero charge." Electrons
will neither leave nor enter the neutrally charged body should it come in contact
with other neutral bodies. If, however, any number electrons are removed from the
atoms a body matter, there will remain more protons than electrons and the whole
body matter will become ELECTRICALLY Positive. Should the positively charged body
come in contact with another body having a normal charge, or having a Negative (too
many electrons) charge, an electric current will flow between them. Electrons will
leave the more negative body and enter the positive body. This electron flow will
continue until both bodies have equal charges. When two bodies matter have unequal
charges and are near one another, an electric force is exerted between them because
their unequal charges. However, since they are not in contact, their charges cannot
equalize. The existence such an electric force, where current cannot flow, is referred
to as static electricity. ("Static" in this instance means "not moving.") It is
also referred to as an electrostatic force.
One the easiest ways to create a static charge is by friction. When two pieces
matter are rubbed together, electrons can be "wiped f" one material onto the other.
If the materials used are good conductors, it is quite difficult to obtain a detectable
charge on either, since equalizing currents can flow easily between the conducting
materials. These currents equalize the charges almost as fast as they are created.
a static charge is more easily created between nonconducting materials. When a hard
rubber rod is rubbed with fur, the rod will accumulate electrons given up by the
fur, as shown in figure 1-5. Since both materials are poor conductors, very little
equalizing current can flow, and an electrostatic charge builds up. When the charge
becomes great enough, current will flow regardless the poor conductivity the materials.
These currents will cause visible sparks and produce a crackling sound.

Figure 1-5. - Producing static electricity by friction.
Q12. How is a negative charge created in a neutral body?
Q13. How are static charges created?
Nature Charges
When in a natural, or neutral state, an atom has an equal number electrons and
protons. Because this balance, the net negative charge the electrons in orbit is
exactly balanced by the net positive charge the protons in the nucleus, making the
atom electrically neutral.
|












