|
August 1932 Radio-Craft
 [Table
of Contents] [Table
of Contents]
Wax nostalgic about and learn from the history of early electronics.
See articles from Radio-Craft,
published 1929 - 1953. All copyrights are hereby acknowledged.
|
Whoa, it's a good thing I read
these articles prior to publishing them, lest some soul unfamiliar with this topic
be lead to the wrong conclusion! Keep in mind that this article was written in 1932,
prior to the development of the quantum mechanical model of the atom, but on the
other hand, Ernest Rutherford and
Niels
Bohr developed their model in 1913, so the relevant information was available.
The
Rutherford-Bohr model of the atom suggested a nucleus comprised
of positive masses called protons, each of which carries a charge of +1 unit, and
neutrons with no net charge. Surrounding the nucleus were orbiting masses called
electrons, each of which carries a charge of -1 units. Accordingly, the net charge
of an atom was the sum of protons and electrons, with unionized atoms having a net
charge of 0 (zero). Neutrons, carrying no charge,
have no effect on the overall atomic charge.
Modern science says quarks, three of which make up each proton and neutron, have
individual charges of +1/3, -1/3, +2/3, or -2/3, thereby determining the particles'
net charges (+1 for protons, 0 for neutrons). Now, take a look at Figure 3 in this
article and the text description that mutually proves the drawing is not a mistake
and the text is not a typo.
The author correctly believes an atomic nucleus (in this case an unionized carbon
atom, N=6), which must have a net charge opposite of the number of electrons (6
for carbon), needs to be +6 charge units. However, he knows the atomic weight (mass)
of a carbon atom is 12 mass units (each proton and neutron has approximately 1 mass
unit). There cannot possibly be 12 protons or that would yield a net atomic charge
of +12 + (-6) = +6 charge units. He resolves the quandary by proposing 6 additional
protons in the nucleus along with 6 additional electrons in the nucleus. That equal
set cancels out the 6 extraneous charge units, and since the mass of electrons is
miniscule compared to that of protons, their presence does not upset known total
atomic mass too much. I don't ever recall seeing that kind of model being proposed
before.
The irony is that the
raison d'être for the article is to push back the frontier of
ignorance so that the reader might more fully understand what is happening in an
electronic circuit. Otherwise, though, it is a useful piece.
In a very loose sense, Mr. Palmer's nucleus model is sort of accurate in
that a neutron, when it decays (beta decay,
mediated by the nuclear weak force), produces a proton, an electron, and an electron
antineutrino (i.e,
free neutron
decay). That is not the same, however, as saying that a neutron is initially
comprised of a proton, an electron, and an electron antineutrino. The process is
not readily reversible.
I Equals E over R

A molecule of a substance, say at B Fig. 1, is composed
of positive and negative electrons, such as illustrated at Fig. 2 or Fig. 3.
Fig. 4 illustrates the logical sequence used in breaking down the substance.
By C. W. Palmer
The fact that current flow in an electrical circuit depends upon voltage and
resistance means nothing unless one can visualize what is actually going on. In
this extremely novel presentation, the author shows not only "how" but "why."
People not familiar with electricity have the idea that little is known about
this subject. This assumption is incorrect, as probably more is known about this
science than about any other. Because mechanical motions and forces can be seen
and felt, it is easy for the average person to understand and foretell their actions
and the results ensuing. For example, few people would question the result of striking
a piece of wood with the sharp edge of an axe or dropping an egg on a concrete floor;
but when the problem is to visualize what is taking place in an electrical circuit,
they are entirely at "sea."
If we remember that we cannot see or hear electricity directly, but can only
observe its effects, the study of electricity - and its companion radio - will be
much simplified.
Electricity (according to the electron theory) consists of extremely small moving
particles, these particles have been named electrons and protons. These electrons
and protons do not carry electricity, as some people think, they constitute electricity.
In other words, an electron or proton is nothing but a small quantity of electricity.
Electrons and protons are separated because they act differently; the electron is
said to be a negative charge while the proton is a positive charge.
The average person usually believes an electron to be a very small particle of
matter; beyond this elementary conception his ideas are vague and usually confused.

The uncharged, separated molecules in Fig. 5 cause a flow
of current as shown in the lower part of the same figure when touched. This flow
ceases in a very short time, but may be caused to flow for a longer time by the
application of an E.M.F. as shown in Fig. 6.
Let us first consider "Matter." Matter is any substance having weight and volume
- the air, the earth, the water, are all forms of matter.
The Atomic Structure
Consider a bar of copper (an element) as shown in Fig. 1. This bar shows
certain peculiarities which identify it as copper, and even a very small piece,
such as B of Fig. 1, cut from this bar will be characteristic of the whole
piece. If it were possible to keep cutting down the size of the piece of copper,
we would arrive at a point where a further cut would result in changing its characteristics,
and it would no longer be identified as the same material as the whole. This particle
containing all the peculiarities of the whole piece is called a molecule of the
element.
Since the molecule has the same characteristics as the whole, it, too, must be
subdivided if we are to discriminate between one substance and another. Now, since
all substances have different constituents, their molecules must be different, and
science has been able to break down the molecule into still smaller particles called
atoms. An atom of hydrogen is different from an atom of helium; an atom of copper
is different from an atom of zinc, etc. Atoms cannot exist by themselves in a normal
state - at least two atoms must be combined to form a molecule.
The atoms of every substance, regardless of its nature, are composed of electrons.
This means that all substances contain electricity, which seems contradictory to
our general knowledge, although it is apparently true as we shall soon see.
In its normal state, an atom contains a certain number of electrons and proton
arranged in a particular manner. Each substance has a different combination and
grouping of the charges. Hydrogen, for example, the lightest substance known contains
only one electron revolving around one proton as shown in Fig. 2. Carbon contains
12 protons grouped together with 6 electrons as a nucleus around which 6 electrons
revolve as shown in Fig. 3.
The central portion of the atom is known as the nucleus and it may consist of
a single proton or a group of protons and electrons. The electrons revolving around
the nucleus are known as the planetary or free electrons, because they can be removed
from the atom without changing its general character. These planetary electrons
revolving around the nucleus may form a single ring or a number of rings around
the nucleus, depending on the complexity of the atomic structure of the substance.
The atomic structure is shown graphically in the form illustrated in Fig. 4
- first, there is the molecule of an element which is composed of atoms and these
in turn are made up of electrons and protons.
Single elements, as described, are familiar to all, but many substances we encounter
consist of chemical combinations of the atoms of two or more different elements
forming another substance - a compound - whose appearance and physical properties
are different from any of the elements, such as salt, water, etc.
For the sake of simplicity, we will limit our explanation to the elements and
atoms.
The Charge
We have shown that atoms are composed of minute charges of electricity which,
normally, are in such a form that the sum of the charges of all the electrons or
negative charges equal the sum of the charges of all of the protons or positive
charges. We have also explained that some of the electrons are revolving around
the nucleus in orbits in a manner similar to the stars around the sun. It is to
be noted that although the substance contains electricity, (electrons and protons)
it is uncharged simply because the charges are equal and balanced.
If we remove one or more of the planetary electrons from an atom, the atom becomes
unbalanced and lacks negative electricity (electrons). In this case, the atom is
said to be positively charged. On the other hand, if we place an additional electron
or electrons in one of the planetary orbits of an atom, it also becomes unbalanced
- in the opposite direction - and has too much negative electricity (too many electrons).
In the latter case, the atom is charged negatively.
From this it can be concluded that a substance is electrified when it has more
or less than its normal number of electrons and the amount of charge is determined
only by the quantity of electrons displaced. Also, it can be deducted that all electrons
are the same regardless of the element or compound from which they come.
The Electric Current
Every substance has a tendency when displaced from equilibrium, to return to
a state of balance as quickly as possible. Just as water will find its own level,
so atoms which have lost electrons (positively charged) will attempt to attach electrons
to themselves, and atoms which have excess electrons will attempt to loose them
and thus become neutral.
Therefore. if we have two substances, one charged positively and the other charged
negatively, and we touch them together; the excess electrons from the negative will
enter the other substance in order to reach a neutral state. If the two bodies are
charged equally (and oppositely) the electrons will continue to transfer until both
substances are neutral.
Figure 5 shows this effect. In the upper part of the illustration the two substances
are charged; and in the lower part, the excess electrons from the negative body
have entered the positively charged body and neutralized the atoms lacking electrons.
If we have some means of maintaining the charges on the two balls (shown in Fig. 5.,)
continuously, there would be a constant passage of electrons from the negative to
the positive ball. This continual passage of electrons is what is known as an electrical
current or simply a current. This follows logically from the statement we made before;
that electrons are electricity.
It is not possible to add or remove electrons from a substance without the aid
of some external force. This force is known as an electromotive force (E.M.F.).
We will not go into the various means of maintaining an E.M.F., here. Several common
sources of electromotive forces are dry batteries, storage batteries and generators.
The amount of current flowing in a circuit (for example the two balls in Fig. 6)
depends on the number of electrons passing through the circuit. The number of electrons,
in turn, depends (among other things) on the amount of the charge which is dependent
on tile E.M.F. applied to the circuit. We may safely conclude, therefore, that the
amount of current flowing in a circuit depends on the value of the E.M.F. applied
to the circuit.
When visualizing the motion of electrons through a solid body, such as copper,
we must remember that the electrons are very small and that there are comparatively
large spaces between the atoms. As an example, if a copper cent were enlarged to
be the size of the earth's diameter, the distance between atoms would be about three
miles and the electrons would be only a few inches in diameter!
Resistance
It is well known that certain materials such as copper, brass, silver, etc. will
readily permit the passage of an electric current, while other materials such as
rubber, mica, porcelain, cotton, silk etc., do not. The former materials are called
conductors and the latter, insulators. The reason why metals are such good conductors
of electricity is that their atoms apparently have a weak attraction for electrons
and large numbers of them are either practically in a free state throughout the
body of the metal or they are easily shifted by any outside electric forces. The
more easily the electrons can be shifted in a metal, the lower its resistance to
a flow of current, merely because a greater current flows for the same value of
applied E.M.F.
This action of resistance in conductors introduces another factor in the consideration
of the strength of current flow. Up to this point we have seen that the amount of
current increases as the E.M.F. increases and since the opposition offered by the
conductor of the current decreases the current, it may be said that the magnitude
of the current flowing in any circuit depends upon the E.M.F. applied and the opposition
offered by the circuit itself.
In order to facilitate the measurement and computation of electric currents,
several units have been set as standards. The E.M.F. is measured in a unit called
a volt; the current is measured in amperes and the opposition or resistance is measured
in ohms. The first of these units is usually represented by the letter E, the second
by the letter I and the resistance by the letter R.
To sum up: the current (number of amperes) flowing in a circuit depends upon
the voltage applied and the resistance of the circuit. To state this in another
way,

A problem involving this condition is shown in Fig. 7. This involves a resistance
of 5 ohms in a 10-volt circuit. Then  or 2 amperes. or 2 amperes.
Another type of problem might arise in which it is desired to know the value
of the resistance in a circuit when the voltage and the current are known. Here
again, Fig. 8 illustrates the conditions. This may be determined from the ratio
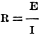 ; or if the potential
(volts) is 50 and the current is 2 amperes, the resistance will be 50/2 or 25 ohms. ; or if the potential
(volts) is 50 and the current is 2 amperes, the resistance will be 50/2 or 25 ohms.
The third condition of the relation considered above is one in which the resistance
and the current are known and it is desired to know the applied potential. In this
case. the voltage E is equal to the product of the current and the resistance (E
= I x R).
If a current of 10 amperes is flowing through a resistance of 20 ohms, then the
potential applied is 10 x 20 or 200 volts.
From these three examples. it is established that there are three individual
conditions involving the relation of E.M.F., current and resistance. These three
classifications are as follows:
When E and R are known and the current is desired:
E = I/R
When E and I are known and the resistance is desired:
R = E/I
When R and I are known and the voltage is desired:
E = R x I
The above three formulas are known as Ohm's Law in honor of the noted physicist
George Simon Ohm.
Resistances in Series
We have already learned that resistance is the opposition of a substance to the
flow of current. It is natural then, that the longer the substance composing the
resistance, the greater will be the value of the resistance. Also, if two conductors
are connected so that the current passes through each of them in succession, then
the resistance of the circuit will be the sum of the individual resistances of the
two conductors. This effect is illustrated at Fig. 8. The resistance of the
conductor at 8A is R. Then the total resistance of the two resistors at 8B is the
sum of the individual resistances.
When the area of a conductor is increased, the opposition to the flow of current
will be decreased, as there are more atoms to lose and gain electrons. It also follows
logically that if two conductors are connected as shown in Fig. 9, the resistance
of the circuit will be less than that of either of the individual resistors R. This
is known as a parallel method of connection.
The method of figuring the total resistance of the circuit for parallel resistances
is different from that for series resistances. If we refer again to Fig, 9, it will
be noted that the current flowing from point A to point B will be divided and part
of it will pass through each resistance. If these resistances are equal, half the
current will go through each, Then, if the applied E.M.F. is 10 volts and the current
in each resistor is 1 ampere, the resistance of each of the resistors will be 10/1
or 10 ohms. However, the total current flowing is 2 amperes, so the resistance of
the parallel circuit is 10/2 or 5 ohms.
For those readers who are familiar with the elements of algebra, the above reasoning
may be expressed in the following formula:
 , etc., , etc.,
in which R is the total resistance and resistors R1, R2, etc., are the individual
resistances of the parallel circuit.
The discussion of electricity and resistance given should be of assistance to
many radio enthusiasts who are confused by the explanations of Ohm's Law usually
given. It is suggested that the article be read over several times so that the details
discussed will all be understood.
(It might be well to add that the current through a given
part of a circuit will vary directly as the applied E.M.F. and inversely as the
resistance, as stated by Mr. Palmer. It should be emphasized, however. that when
part of a circuit is under consideration, the current, voltage and resistance of
that particular part should only be considered, regardless of whatever else occurs
in another part of the circuit. - Editor.)
|







 or 2 amperes.
or 2 amperes. 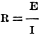 ; or if the potential
(volts) is 50 and the current is 2 amperes, the resistance will be 50/2 or 25 ohms.
; or if the potential
(volts) is 50 and the current is 2 amperes, the resistance will be 50/2 or 25 ohms.
 , etc.,
, etc., 

