|
January 1973 Popular Electronics
 Table of Contents Table of Contents
Wax nostalgic about and learn from the history of early electronics. See articles
from
Popular Electronics,
published October 1954 - April 1985. All copyrights are hereby acknowledged.
|
This 1973 Popular
Electronics magazine article has got to be one of the most concise and interesting
summaries published of Alessandro Volta's path to the discovery of battery cells,
aka "piles." Author David Heiserman covers not just the technical aspects of Volta's experiments, but the
personal and political tip-toeing he needed to endure with contemporary physicist
Luigi Galvani and conqueror Napoleon Bonaparte, respectively. Alas, that has been
the case throughout history regarding not offending certain powerful people. Although such unnecessary struggles continue to impede
scientific advancement, at least the unfettered access to the Internet has provided
a venue for otherwise undiscoverable work to be aired. Today, though, while you
might not be tortured, imprisoned, or murdered for your work, you can still be
"cancelled" through delisting, denying access to funds, blocking, and any number
of other nefarious tactics.
Volta and His "Electric Pile"
By David L. Heiserman
 When Alessandro Volta, Italian physicist
and protégé of Napoleon Bonaparte, announced the invention of his "electric pile"
in 1800, he touched off an explosion of scientific discoveries and technological
innovations that have shaped the world we know today. Volta's pile, forerunner of
modern primary-cell batteries, was a revolutionary device because it was the very
first source of continuously flowing electrical energy. When Alessandro Volta, Italian physicist
and protégé of Napoleon Bonaparte, announced the invention of his "electric pile"
in 1800, he touched off an explosion of scientific discoveries and technological
innovations that have shaped the world we know today. Volta's pile, forerunner of
modern primary-cell batteries, was a revolutionary device because it was the very
first source of continuously flowing electrical energy.
Just prior to Volta's announcement, scientists were preoccupied with the problems
of understanding and applying the only kinds of electricity known at the time -
static electricity and the mysterious "animal electricity" discovered by Luigi Galvani.
Volta's pile changed the course of electrical research, leaving the brief sparks
of static electricity behind as a specialized branch of physics and making Galvani's
discoveries into nothing more than a scientific curiosity.
As a professor of physics at the Italian universities at Como and Pavia, Volta
spent the first thirty years of his career looking for ways to generate, measure,
and control static electricity. His electrophorus and condensing electroscope both
evolved directly from this line of work. When Galvani sent him a copy of a paper
describing a new kind of "animal electricity," Volta immediately dropped his work,
and began reproducing the famous frog-muscle experiments. His primary objective
was to help Galvani explain his strange observations.
Galvani, an Italian physician in Bologna, had accidently discovered that touching
the nerves in a frog's leg with a pair of unlike metals made the muscles convulse.
He thought that the living tissues, and not the metals, were the source of electrical
energy. Galvani called this form of electricity "animal electricity" to distinguish
it from static electricity, and he sincerely believed he had uncovered the secret
force of life.
According to Galvani, the nerve tissue generated the electrical energy that made
the frog's leg convulse. In the light of modern electrophysiology, he wasn't far
from wrong. Where Galvani went off track was with his belief that the metals merely
completed the circuit between nerve and muscle. As a methodical experimenter, Volta
tried the experiment using two pieces of the same metal - something that never occurred
to Galvani. The frog's leg did not twitch. The discrepancy came as a big surprise
to both men. Volta responded by re-evaluating Galvani's theory. Galvani and his
followers responded by criticizing Volta's new-fangled experimental procedures.
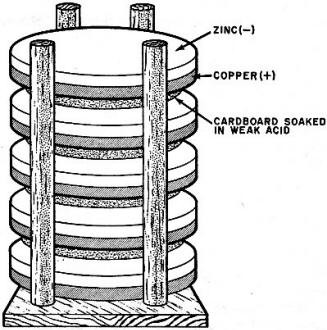
Lower part of Voltaic pile. Most had 30 sets of metal and cardboard
discs.
At the time, it took a great deal of bold and creative insight to propose that
the metals, and not the animal tissues, were behind this new kind of electrical
energy. When Volta arrived at this notion, he began a series of experiments to show
that a pair of unlike metals can produce electricity without the help of any kind
of animal tissues. His first experiments used a single pair of metals bathed in
either a brine solution or a dilute acid. Volta, by the way, was never able to explain
the function of the brine or acid.
When Galvani heard about Volta's new experiments, he responded by pointing out
the fact that an electric eel can produce large amounts of "animal electricity"
without the help of any kind of metal. For the time being, this argument stumped
Volta; but it didn't stop him.
The only research instruments at the time were those designed for studying static
electricity - hardly the kinds of instruments a modern-day technician would use
to study the nature of batteries. Thus, Volta had to juggle electroscopes, charged
glass rods and slabs of resin to measure his "metallic contact electricity."
Volta determined the relative intensity of his electrical forces by charging
an electroscope with his brine-bathed metals. The farther the leaves separated,
the larger the electrical tension. He determined the polarity of his potentials
by first charging an electroscope with a static charge of known polarity, then touching
it with one of the metallic electrodes. If the leaves fell together, the electrode
had a charge opposite that of the reference static charge. If the leaves separated
even farther, the polarity had to be the same as the reference static charge.
His first experiments showed that different combinations of electrode metals
produced different polarities and amounts of electrical tension. By mating all possible
pairs of electrodes made from lead, zinc, copper, graphite, silver and gold, Volta
constructed the first table of electromotive elements. He and other investigators
later used this table to predict the voltages and polarities a certain combination
of metals would produce.
While Galvani was quietly gathering supporting data for his findings, Volta had
to work between bouts of accusations and "public insults" concerning his allegiance
to Italy. When Napoleon and his armies stormed into northern Italy, making Volta's
state of Lombardy part of the French Empire, Volta was one of the Italians sent
to greet the noted conqueror. Being a resident of the little state, Volta wasn't
overly enthusiastic about his mission; but having met Napoleon in Paris several
years before, Volta decided it would be wise to move with the tide of the times,
and make the best of the political situations around him.
Volta wasn't a traitor in the usual sense because the people of Lombardy had
always been sympathetic to the French cause. They looked upon Napoleon as one who
could finally unify the separate states of their nation. Nevertheless, a few influential
Italians living in other parts of the country began making trouble for the physicist.
To add fuel to the animosities between Volta and Galvani, the physician stubbornly
refused to pledge allegiance to the French flag. Volta's situation became so bad
at one point that Napoleon, himself, intervened to save the neck of his favorite
scientist.
When the political air finally cleared, Volta returned to the scientific tasks
at hand. The only remaining problem was finding a way to get a more convincing amount
of electrical tension from his contact metals. He came upon the idea of piling copper
and zinc discs on top of one another, sandwiching a piece of brine-soaked cardboard
between each pair. This "pile" of metals and cardboard actually formed what we now
know as a series aiding circuit.
In 1800, Volta described the final results of his work in a long, two-part letter
to Joseph Banks, President of the Royal Society of London. Banks prepared the letter
as a formal scientific paper for the Philosophical Transactions of the British Royal
Society. Published under the title of "On the Electricity Excited by the Mere Contact
of Conducting Substances of Different Kinds," the world learned of Volta's source
of continuously flowing electrical energy.
This famous paper clearly showed that a chemical action between pairs of brine-
or acid-soaked metals produced the new form of electricity. Thus, Galvani's theory
suffered a sudden and complete death.
The first part of Volta's paper described the construction and effects produced
by his electric pile: "... if the sets of triplets of the plates be added 20 or
30 more, disposed in the same order, the actions of the extended pile will be much
stronger, and be felt through the arms up to the shoulders; and by continuing the
touchings, the pains in the hands become insupportable."
Volta also noted that the "perpetual" electrical action stopped as the cardboard
pieces began to dry out. To remedy this problem Volta invented the "crown of glasses"
- glasses filled with a weak acid solution and containing a pair of unlike metals.
By attaching wires from an electrode of one kind to an electrode of the other kind
in another glass, Volta wired up what we now know as a series cell arrangement.
This makes up a more familiar version of wet-cell batteries that are employed today.
Although Volta and Galvani were bitter enemies, Volta gave Galvani full credit
for leading him into the kind of research that resulted in the "pile" and "crown
of glasses." Largely due to Volta's support, his "perpetual" current became known
as "galvanic current." Electrical researchers later named the galvanometer after
the unfortunate Italian physician.
Volta, however, could not resist taking one public jab at the man who had been
his rival for so many years. When Napoleon invited Volta to demonstrate his inventions
before the political and scientific leaders of the French Empire, Volta introduced
his electric pile by unwrapping it from the skin of an electric eel - the animal
that represented Galvani's strongest argument for "animal electricity."
Posted February 28, 2024
(updated from original
post on 11/6/2017)
|









