|
September 1964 Popular Electronics
 Table
of Contents Table
of Contents
Wax nostalgic about and learn from the history of early electronics. See articles
from
Popular Electronics,
published October 1954 - April 1985. All copyrights are hereby acknowledged.
|
When you think of typical
primary battery cells (non-rechargeable by definition), something like the standard
Ray-O-Vac carbon (actually zinc-carbon) model probably comes to mind. The reason
primary cells cannot be recharged is that the cathodes are consumed in the reaction
with the electrolyte during current flow. Secondary cells are rechargeable because
the current-producing reaction does not consume the cathode (at least not as rapidly),
so applying a reverse voltage drives the electrons back from whence they came allowing
the discharge process to happen again. There is another type of primary cell - the
fuel cell - that never really discharges but is constantly fed with a chemical (or
combination of chemicals) that facilitates a reaction between electrodes and the
electrolyte. Therefore it never needs to be recharged in the traditional sense -
just refueled. In a sense a fuel cell is more of an electric generator than a battery.
Turn off the external energy source (coal, hydro, nuclear, wind, sunlight) to a
generator and the current flow stops. This report from a 1964 edition of Popular
Electronics describes some of the early work on fuel cells.
The Fabulous Fuel Cell

New electrode materials are constantly being evaluated in the
search for reliable, economical fuel cells. At left, a G.E. researcher tests electrode
material with electrolytes of varying acidity and alkalinity.
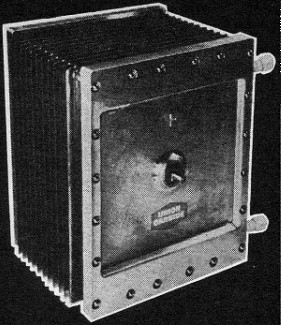
The 12" x 14" fuel cell module uses economical carbon electrodes
combined with a minimum of precious metal catalyst. Made by Union Carbide, it is
a hydrogen-oxygen low-temperature unit.
By Walter G. Salm
Even as you read this, the first fuel cells are on their way into space. Tomorrow?
Fuel cell-powered cars are just one possibility.
One day in the not-too-distant future you may be able to drive into a gas station,
pull alongside a pump labeled "methane," and order a tankful for your car.
You won't be driving some new breed of jet or turbo-powered chariot, but a car
with a power plant that is as old as the automotive industry itself - an electric
motor. The unusual feature of this car will be the part that provides the electricity,
a new kind of generating device that gulps a variety of inexpensive gases and produces
power. The device is called a fuel cell, and while it is still experimental, companies
working on its development have already reported progress that seems almost unbelievable.
This generalized drawing shows basics of fuel cell operation. The electrolyte
may be liquid, semiliquid, or solid; the electrodes can be carbon, plastic, platinum
or nickel boride, typically in combination. Fuel depends largely on the type of
electrode used; hydrogen reacts very easily, but inexpensive hydrocarbons are now
being used thanks to improvements in cell electrodes.
The fuel cell is a kissing cousin of the more conventional electrochemical batteries
that we use every day. Like batteries, it works through a chemical reaction that
produces a lot of free electrons. But unlike batteries, it can be "refueled" by
replacing chemicals that have been consumed in the reaction, and it will continue
to function at normal operating levels all the while. And what operating levels
they are! Fuel cell modules have already been constructed with continuous outputs
of 2.5 kilowatts. When discussing characteristics and life tests, it is customary
to refer to a cell in terms of amperes-per-square-foot (of electrode surface), and
these figures are normally several hundreds of amperes for a typical fuel cell configuration.
To understand just what all the noise is about, let's take a quick look at conventional
batteries and the way they produce electricity. Dry cells, whether of the zinc-carbon
type used in flashlights or the more sophisticated alkaline variety, all produce
electric current by means of the chemical reaction that goes on between certain
key materials - the electrodes and the electrolyte. The electrolyte is a liquid
or semiliquid that reacts chemically with the negative electrode, usually zinc,
producing many free electrons. The electron stream returns through the load to the
positive electrode and moves through the electrolyte to the negative electrode where
the electrons are again freed by the chemical action.
This chemical action consumes both the negative electrode and the electrolyte.
In dry cells, the result is a dropping off of the cell's productivity; eventually
the cell must be discarded. In wet-cell batteries such as the automotive type, if
the consumption of negative electrode and electrolyte has not progressed too far,
the chemical action can be reversed by applying a direct current to the battery
terminals to recharge it. The ability to be recharged draws a distinct line between
two battery types. Primary batteries cannot be recharged; secondary batteries can.
Enter the Fuel Cell.
Although there are many similarities between a fuel cell and a primary battery,
the big difference is that the electrodes and electrolyte used in the fuel cell
are not changed or consumed during the operating life of the device.

A hydrogen-oxygen fuel cell, circa 1959, shows immense progress
that has been made in a few years. Object at right of G.E. cell is a motor with
a propeller.

A recent development is a cell that uses inexpensive hydrocarbon
fuels and oxygen. Devised by Dr. Thomas Grubb and Dr. Leonard Niedrach of G.E.,
the cells shown below operate on such fuels as diesel oil, gasoline, and propane
gas.

This experimental high-temperature fuel cell uses a solid zirconia
electrolyte (white cylinder). The dark cylinder is a graphite electrode. Cell uses
natural gas and oxygen to generate electricity.

A cutaway mockup of one of the fuel cell canisters installed
in Gemini. Fuel cell modules - the first may be in orbit when you read this - produce
drinking water for astronauts as well as up to two kilowatts of electrical power.

This fuel-cell-driven golf cart made by Allis-Chalmers shows
the feasibility of putting fuel cells to work powering vehicles. A fuel-cell-operated
farm tractor was demonstrated by firm as early as 1959.

A classroom demonstrator of fuel cell principles, this working
model created by Allis-Chalmers uses either alcohol or sodium or potassium hydroxide
as fuel, and hydrogen peroxide as an oxidant. Platinum, silver, and nickel electrode
plates are positioned in tank at right, and the two end plates connected to the
miniature electric motor furnished with the model. Priced at $9.75, model is available
from Science Materials Center. Inc., 220 East 23 St., New York, N.Y. 10010, less
necessary fuels.
The zinc (or magnesium or lead) electrode used at the anode in a primary battery
cell actually serves two purposes - that of an electrode and that of a "fuel" which
is consumed as the cell wears out. The electrodes used in a fuel cell are not used
as fuel. The fuel-hydrogen, hydrocarbons, etc. - is continuously fed to the cell
from an external source.
As shown in the generalized drawing of a fuel cell (p. 48), the chemical reactions
that produce a flow of electrons in the external circuit take place in the cell's
porous catalytic electrodes. This terminology largely explains the function of the
electrodes: they absorb fuel and oxidant by virtue of being porous, and promote
a reaction between the two which generates electricity. Producing the perfect electrode
for fuel cells is one of the big problems that has stumped researchers ever since
a brilliant scientist, W. R. Grove, conducted experiments with elementary fuel cells
back in 1839. Carbon and polymer plastics have been used for electrodes. More recently,
spongy platinum and nickel boride have come along. Without laboring the point, producing
economical electrodes that can promote and contain fuel cell reactions without themselves
changing is no mean trick.
In operation, the two electrodes of a hydrogen-oxygen fuel cell absorb their
gases by diffusion, the anode taking on oxygen and the cathode hydrogen. The two
electrodes are separated by a liquid or solid electrolyte, and the reaction takes
place at the surface where the electrolyte makes contact with the electrodes.
When the oxidant (air or oxygen) reaches the cathode of the fuel cell, it is
absorbed by the cathode and enters the electrolyte in a process called "sorption."
The exact mechanism by which sorption (a general term meaning the same as "absorption"
but used when the phenomenon is unknown or indefinite) of the oxygen takes place
remains one of the mysteries of fuel cell operation. On reaching the anode, the
oxygen combines with the fuel absorbed by the anode and oxidizes it, producing electricity
in the process.
Amazing as it may seem, no heat is produced other than a small amount due to
electrochemical inefficiencies. The fuel cell thus becomes the world's most perfect
generator of electricity. With no. moving parts and no energy-wasting boiler-turbine
combinations which convert fuel by burning it, the fuel cell strips electrons from
the fuel and sends them into an external circuit to do useful work!
Waste Products.
Referring back to the fuel cell drawing, the stripped atoms of fuel, now positive
ions, migrate back through the electrolyte to the cathode where they combine with
the oxidant to produce water, a "waste" product which, incidentally, may prove very
useful. Depending on the fuel used a waste product is also produced on the anode
side of the fuel cell; in the case of hydrocarbons, this is carbon dioxide as in
a gasoline engine. Unlike a gasoline engine, however, which may have at most a conversion
efficiency of 30 to 40 percent, the fuel cell has efficiencies of 50 to 60 percent
at present, and theoretical levels up to 98 percent.
Another big fuel cell advantage is that air can be used as the oxidizing gas.
This completely eliminates the need for a separate oxygen supply for cells operating
anywhere on the earth's surface. Of course, cells lofted into outer space must carry
their oxygen. The one disadvantage of using air is the lower productivity that results.
When a cell is pressurized, the available yield in amperes per square foot of electrode
goes up. As the device operates, its temperature also goes up (due to the inefficiencies
mentioned earlier) which further raises the yield.
While fuel cells using inexpensive hydrocarbon fuel (i.e., anything from natural
gas to gasoline to diesel fuel) hold the most promise for future down-to-earth commercial
applications there is still a great deal of developmental work ahead. One of the
major obstacles is the high cost of the platinum alloy electrode material which
seems to hold the key to making these inexpensive fuels react to produce electricity
in a fuel cell.
High-Temperature Cells.
Raising the operating temperature raises the cell's output, but with one bad
side effect - it causes corrosive action at the electrodes, a condition that can
ruin the cell after a relatively short time. But the advantages of elevated temperatures
can be retained by the use of a solid electrolyte designed to withstand them. One
such material in use is lime-stabilized zirconia.
In a cell of this type, a fuel such as methane (natural gas) is fed to one side
of the cell where it forms carbon on the electrolyte surface. The carbon becomes
both the anode and the fuel. The operating temperature of this cell is normally
about 1800° F (about 985° C). This temperature is above the melting point
of silver and it is molten silver which forms the base for the negative electrode.
Oxygen is diffused into the silver, and the high operating temperature is maintained
simply by burning off gases within the cell. High-temperature cells in this category
have produced current densities up to 150 amperes per square foot of electrode area.
Nominal voltage for such a cell is 0.7 volt, making the single-cell power output
a little over 100 watts per square foot of electrode.
A further development that is still being evaluated is known as the "Redox" (reduction
and oxidation) cell. This device involves a two-step process in which an intermediate
gas-liquid reaction occurs in the electrolyte itself. The Redox cell, although it
isn't as efficient as the more conventional types, has lower internal resistance
losses which more than offset the lower efficiency level. It is still largely experimental,
however.
Fuel Cells in Outer Space.
The state of the art has advanced sufficiently in certain cell types to make
it possible to use fuel cells in space vehicles. Several experimental devices have
been lofted into outer space as part of a testing and evaluation program. The units
tested have shown virtually no effects from prolonged periods of weightlessness
and high-gravity acceleration and deceleration. Cells recovered from space probes
have continued to function normally in laboratory life tests, still operating at
optimum efficiency.
In fact, the space testing has been so successful that G.E. is now building fuel-cell
modules for use in the Gemini space program at the rate of one complete system every
two weeks. The first systems have been delivered, and one is scheduled for launching
later this year - perhaps even as you read this - as part of the equipment of the
unmanned Gemini Number Two space vehicle.
The Gemini system is made up of twin canisters two feet long and a foot in diameter,
each containing 100 individual solid-electrolyte (the electrolyte portion of each
cell is known as an "ion-exchange membrane") fuel cells. The system is highly reliable,
has a high power output (up to two kw.); and is much lighter in weight (145 pounds
not counting fuel) than any other comparable power source.
By way of comparison, a typical fuel cell system designed to provide outputs
of 500 watts to two kilowatts for 10,000 hours reliability weighs (including fuel)
between 400 and 500 pounds. Solar cells and battery systems with comparable outputs
and reliability would weigh in the neighborhood of 700 pounds. And solar cells have
a further disadvantage. Because they must be mounted externally on the space vehicle,
they are especially susceptible to damage by radiation and minor meteor collisions.
The twin cylinders installed in Gemini Two each contain three fuel-cell stacks
which can be operated separately depending on power supply requirements. The fuels
are stored at temperatures near absolute zero, and waste heat generated within the
cell is carried off by a circulating cooling system. Another aspect of the fuel
cell is its by-product: potable water. In Gemini, the water will be made available
for consumption by the astronauts who man future vehicles, thereby reducing the
payload.
Military Applications.
Compared with conventional power sources in size, weight, and maintenance required,
the fuel cell offers some enormous advantages. In a typical military field application,
such as providing power for a front-line communications outpost, the fuel cell is
expected to surpass such power sources as primary batteries, secondary batteries
including NiCads and wet-cell storage types, and the frequently used gasoline-driven
motor-generator.
The primary batteries have to be replaced frequently, especially if they must
deliver sustained current outputs for radio transmission.
Secondary batteries must be recharged. This means using a noisy (and therefore
frequently undesirable) motor generator set or replacing the batteries at regular
intervals with recharged units brought up from the rear. The motor generator itself
may be too cumbersome to bring up to some positions, its noise of operation can
attract the enemy's attention, and it must be constantly pampered, fueled and maintained.
The fuel cell is completely quiet in operation. It can deliver sustained high
current for indefinite periods of time, and it is fueled with easily transported
gases or liquids. In fact, the total weight of a fuel cell system along with enough
fuel to run it for several weeks may be less than the weight of a comparable set
of storage batteries that require constant recharging.
And powerful they are. On the basis of present-day technology, fuel cells will
soon be able to deliver about a kilowatt for every 15 to 20 pounds of weight! Yet
another advantage of fuel cells as compared to gasoline engines, for example, is
that fuel cell efficiency increases with partial loads, and under no-load conditions,
no fuel is consumed at all. This no-load no-consumption feature separates the fuel
cell from both engines and conventional electro-chemical batteries. Any engine uses
fuel when it is idling.
Future Uses
Earthbound applications for fuel cells in the near future include providing power
for electric switching locomotives; experts believe that such an all-electric system
will be far more efficient and easier to control than the conventional diesel-electrics
in common use today. Powering midget submarines is another potential application,
although the subs will have to carry a canned oxygen supply for extended periods
of deep under-surface travel; a snorkel will provide air for shallow operation.
One of the most intriguing possible uses is in the electric automobile. Several
years ago, a major manufacturer of solar cells exhumed a museum-piece electric car
and covered its roof with solar cells as a publicity stunt. The car ran beautifully
as long as the sun was shining. What was possible with primitive turn-of-the-century
batteries and today's solar cells will certainly be feasible with fuel cells. If
the car's cells use methane, the car can be refueled simply by having the local
power company run a pipe for natural gas into the garage. Refueling on the road
will be done the same way, via natural gas outlets in filling stations. And it'll
be a lot cheaper than gasoline. There will be far less maintenance required, too,
since an electric motor has just one moving part.
As a portable source of direct energy conversion, the fuel cell appears to hold
almost unlimited promise. Its ruggedness and reliability have already been proven
in the rigorous environments of outer space and re-entry, and continuing tests indicate
an almost incredible life-span for this electrochemical generating device.
Posted September 7, 2020
(updated from original post on 8/15/2013)
|















