|
Webster's dictionary
defines luminescence as, "the low-temperature emission of light produced
especially by physiological processes (as in the firefly), by chemical action,
by friction, or by electrical action." Per this article on electroluminescence
in the November 1961 edition of Popular Electronics, Webster defined
it back then as, "any emission of light not ascribable directly to incandescence,
and therefore occurring at low temperatures." Interesting that the contrast
to incandescence is no longer used. Maybe in 1960 the phenomenon of electroluminescence
was still new and novel enough to emphasize the distinction. Barely a decade
had passed since it had moved out of the laboratory and into the marketplace.
The ability to economically produce large panels for lighting was deemed groundbreaking,
and indeed it was. Today, our large screen TVs rely heavily on the technology.
Now, as in 1961, a relatively short useful lifetime plagues electroluminescent
display.
Electroluminescence
 At one time only a dream of science, heatless
illumination is now a reality. Electroluminescence is being put to work to make
paper-thin panels which someday may light your home. At one time only a dream of science, heatless
illumination is now a reality. Electroluminescence is being put to work to make
paper-thin panels which someday may light your home.
By James E. Pugh, Jr.
In early times, the glowing tree-trunk seen by a lone traveler in the woods
or the strange "burning" of the sea noticed by a wide-eyed sailor seemed to
portend evil and disaster. Today we know that both are caused by chemical reactions
in the cells of tiny living things, and our superstition has changed to active
interest. Scientists have been fascinated by the heatless light produced by
certain organisms for decades but, until recently, they couldn't even come close
to duplicating it on a practical scale.
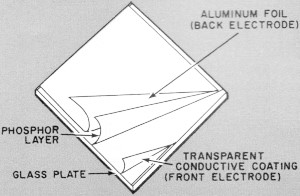
Sandwich-like construction of an electroluminescent lamp
can be seen in this simplified drawing. The front plate has a transparent conductive
coating which acts as an electrode, yet passes light.
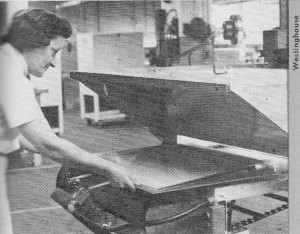
The manufacturing equipment, in theory, imposes the only
limitation on the size of the lamps. Here, a phosphor layer is being baked
in an infrared oven.
The introduction of the fluorescent lamp (1938) was a step in the right direction.
Here, less power was wasted in the production of useless heat than with an incandescent
bulb, though the loss was still substantial. In the late 1940's, however, the
phenomenon of electroluminescence, a direct conversion of electricity to a "cold"
light much like that found in nature, moved out of the laboratory. Today you
can buy an electroluminescent lamp for a few cents in any hardware store.
But just what is electroluminescence? Well, Webster defines luminescence
as "any emission of light not ascribable directly to incandescence, and therefore
occurring at low temperatures." Generally speaking, luminescence is caused by
excitation of the electrons in a chemical compound called a "phosphor." In the
case of living things which luminesce, the excitation is brought about chemically
and the phenomenon is usually referred to as chemiluminescence. Therefore, electroluminescence,
as you might guess, is luminescence produced by a phosphor which has been excited
electrically.
Electrical "Sandwich." Though electroluminescent lamps are not yet efficient
enough to provide general lighting (being most commonly used as night-lights),
they have some unique physical and electrical characteristics which suggest
many uses. The lamps are made by sandwiching a layer of phosphor (often zinc
sulfide) between two plates of electrically conducting material. The front plate
usually consists of glass having a special transparent coating which allows
it to act as an electrode, and the. complete unit is not much thicker than that
plate.
When an alternating current is connected across the two plates, the electrons
in the phosphor are excited to a higher energy level - causing it to emit a
soft, glareless light with almost no accompanying heat. The color of the light
depends on the selection of the phosphor and the impurities which are deliberately
introduced into it. Green, blue, orange, yellow, gold, red, and white phosphors
are available, but at present green yields the most light.
>Contrast the way in which light is produced by an electroluminescent
lamp with the operation of standard fluorescent or incandescent units. Though
a phosphor is also used in the fluorescent lamp, it is not excited directly
by an electric current - but rather by ultra-violet rays. These rays are produced
(along with wasted radiations at other frequencies) by an electrical discharge
through mercury vapor gas. In the case of the incandescent lamp, of course,
the light is only a byproduct of the heat generated in a fine wire by the passage
of an electric current.

Graphs show brightness versus frequency (left) and voltage
(right) for lamps of different ratings.

Typical Characteristics of Electroluminescent Lamps
| Typical Characteristics
of Electroluminescent Lamps |
| Operating Voltage |
Available in standard voltages from 120 to 600 volts |
| Operating Frequency |
Most useful range: 25-1000 cycles |
| Resistance (of a square foot) |
24,000-55,000 ohms, depending on voltage rating |
| Capacitance |
0.35-0.109 uf./sq. ft., depending on voltage rating |
| Operating Current (@ 120 v., 60 cps) |
16-36 ma./sq. ft. |
| Light Output (@ 120 v., 60 cps) |
0.5-1.9 footlamberts |
| Life Expectancy (@ 120 v., 60 cps) |
5000-20,000 hours |
Advantages and Drawbacks. The new light source is usually
produced as a flat sheet, but it can be cut into almost any conceivable shape.
And, since no vacuum is required for its operation, the only limits to its physical
size are those imposed by the manufacturer's equipment. One unit recently made
by Sylvania measures eight by two feet - a whole wall could be made to glow
with a soft, pleasant light by installing a number of these panels side by side.
Freedom from "burn out" is one feature of the new light source. Light output
simply diminishes with time as illustrated in this graph of a typical lamp operating
at 120 volts, 60 cycles. One manufacturer's conservative estimate of the maximum
usable life of his product under these operating conditions is 5000 hours. Another
maker claims up to 20,000 hours of useful operation.
The electroluminescent lamp's main drawback is its present inability to produce
enough light for high-intensity applications. The light output of a given phosphor,
however, can be increased by raising its supply voltage and/or frequency. Though
120-volt, 60-cycle operation is common, special oscillators have been used to
provide higher frequencies (up to 20,000 cycles), and lamps rated at voltages
up to 600 are readily available. An interesting idiosyncrasy of these lamps
is that, with some phosphors, a color shift occurs as the frequency is raised.
In the case of one green phosphor, the color changes to blue at frequencies
over 400 cycles.
It will be a while before you can light your house with glowing ceiling or
wall panels but, in the meantime, electroluminescent lighting will find plenty
of applications. In addition to their other advantages, these "miracle" lights
have a usable life expectancy of up to 20,000 hours. During this time a burn-out
never occurs - the light intensity simply decreases slowly until it reaches
a level so low that the unit must be replaced. Then, too, power consumption
is quite low. A Westinghouse "Rayescent" lamp, for instance, draws only 0.6
watt per square foot when operating at 120 volts, 60 cycles.
Present and Future. Long life, freedom from "catastrophic" burn-out, and
glareless light make these lamps particularly well suited for use as indicators
of all kinds. Among the applications in this category which have been suggested
or already developed are lighting for aircraft, auto, and boat instrument panels,
highway signs, and "read-out" numbers.
Interior decorators, too, are excited by the possibilities of this flexible
light source. Glowing walls and ceilings (primarily for decorative effect rather
than illumination) are already a reality, and electroluminescent panels have
been incorporated into many types of furniture. Perhaps in the not-too-distant
future this revolutionary lighting material will be improved to the point where
it can be used for general lighting as well as decoration - and you may have
to look in an antique store to find one of today's "old-fashioned" light bulbs.

What may be the world's largest electroluminescent lamp
measures eight by two feet. It has a brilliance of five foot-lamberts, an estimated
life of five years.
Electroluminescent lamps are well suited for use as indicators of all kinds.

Electroluminescent street sign.


Night-and-day photos of a tractor dashboard incorporating
the new light source.

Table top emits a soft "candlelight" glow flattering to
diners.
Posted March 19, 2021
(updated from original post on 9/2/2012)
|




 At one time only a dream of science, heatless
illumination is now a reality. Electroluminescence is being put to work to make
paper-thin panels which someday may light your home.
At one time only a dream of science, heatless
illumination is now a reality. Electroluminescence is being put to work to make
paper-thin panels which someday may light your home.









