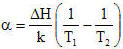|
The Arrhenius equation
predicts failure acceleration rate due to temperature increase. Although originally developed to describe chemical
reactions due to temperature, it applies equally well to electronic assembly failure rates. The Arrhenius
activation energy, ΔH, is all that is needed to calculate temperature-related acceleration. Swedish chemist
Svante Arrhenius provided a physical justification and interpretation for his observation back in 1899. His
equation can be used to model the temperature-variance of diffusion coefficients, population of crystal vacancies,
creep rates, and many other thermally-induced processes/reactions. A useful generalization borne out by the
Arrhenius equation is that for many common chemical reactions at room temperature, the reaction rate doubles for
every 10 °C increase in temperature.
| F = |
x1
x2 |
= eα
{Lifetime Acceleration Factor} |
| Where: |
x1 = Failure rate at junction temperature T1 x2 = Failure rate at junction
temperature T2 T = Junction temperature in degrees K ΔH = Thermal activation energy in eV k =
Boltzmann's constant |
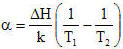 |
|