|
November 1969 Electronics World
 Table of Contents
Table of Contents
Wax nostalgic about and learn from the history of early electronics. See articles
from
Electronics World, published May 1959
- December 1971. All copyrights hereby acknowledged.
|
Galvanic corrosion is
a potential problem (get it - potential?) for just about any scenario where two
metals of differing nobility (position the galvanic table) come into contact
with each other. This 1969 Electronics World magazine article explains
corrosion as an electrochemical process akin to electronics, involving anodes,
cathodes, electrolytes, and electron flow, particularly in marine environments.
Galvanic corrosion occurs with dissimilar metals like zinc (anode) and copper
(cathode) in water, accelerated by oxygen depolarizing the cathode. Factors
include galvanic/activity series rankings, electrolyte pH, chloride content,
humidity, and oxygen differentials causing pitting. Electrolysis arises from
stray currents or voltage drops in boat wiring; local-cell action from metal
impurities. Prevention strategies include selecting compatible metals, using
zinc sacrificial anodes, or impressed-current cathodic protection systems with
reference electrodes and platinum anodes to counter galvanic action. Instruments
like pH meters, voltmeters, and chloridometers aid diagnosis, emphasizing
electronics technicians' role in marine corrosion control.
The Electronics of Corrosion

Fig. 1 - A portable battery-operated pH meter that is used by
electronics technicians who specialize in marine work.
By William P. Ferren, Ph.D.
F.A.I.C.* /Associate Professor of Chemistry
Wagner College, N.Y.
The role played by electronics in causing and/or minimizing corrosion, especially
as related to marine environments, is explained. The various types of corrosion,
as dependent on the environment (electrolyte) and the galvanic arrangement (dissimilar
metals), are discussed by author.
The fundamental action in corrosion is electronic in nature. This electro-chemical
process holds true whether the corrosion takes place in an air environment, in the
water, or in the interface between the two. Indeed, much of the terminology used
by corrosion scientists and engineers reflects this. Such terms as anode, cathode,
electrolyte, ionization, current, potential, voltage and e.m.f. are as familiar
to the corrosion engineer as they are to the electronics technician.
Galvanic Corrosion
Similarly, the classical explanation of the phenomenon of corrosion is readily
understandable by those in electronics. Any metal placed in a water solution will
begin a process of dissolution. If we take two unlike metals such as zinc and copper,
place them in water, and wire them together external to the water electrolyte, we
have created conditions whereby corrosion can take place. It is from this simple
galvanic cell that the particular type of corrosion that occurs when two dissimilar
metals are exposed to a common electrolyte and are physically connected externally
derives its name - galvanic corrosion. The zinc atoms in contact with the water
lose electrons and enter the water as positively-charged zinc ions. The electrons
released by the ionized zinc atoms will travel through the wire from the zinc anode
to the copper cathode. In the electrolyte, the zinc ions will travel toward the
copper cathode. But these zinc ions never reach the copper cathode because of a
chemical reaction that has been taking place within the electrolyte. The water atoms
have also been ionizing; the hydrogen atoms in the H20 combination become
positively charged hydronium ions by giving up electrons, and negatively charged
hydroxyl ions by gaining electrons. The positive hydronium ions seek to regain their
lost electrons and take them from the copper cathode which by now has an abundance
of the electrons released by the zinc atoms when they ionized. Thus, the hydronium
ions become hydrogen molecules when they achieve balance. The negative hydroxyl
ions share their excess electrons with the zinc ions and here, too, balance is achieved.
If nothing further happened ill this system, the galvanic corrosion process would
cease for the hydrogen atoms clustered around the copper cathode in the form of
hydrogen gas (polarization) would effectively shield it from the electrolyte. But
oxygen becomes involved and combines with the hydrogen atoms to form water -Ho()
-once more. This action exposes the copper cathode to the electrolyte, thereby re-
establishing the electrical circuit, and permitting the corrosion process to continue.
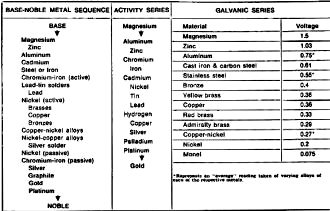
Table 1 - Listings of base-to-noble metal sequence, activity
series, and galvanic series. Base metals at the top of the list function as the
anode when used with metals lower in the series (more noble), and are subject to
corrosion. The activity series, with hydrogen gas as the arbitrary reference, indicate
the relative inertness of reactivity of metals. The reactive elements are above
hydrogen while the inert elements are below. The galvanic series, the most used
series in considering the electronics of corrosion, indicate voltage readings recorded
between the indicated metal and a silver/silver-chloride reference electrode while
immersed in a relatively unpolluted sea-water electrolyte.
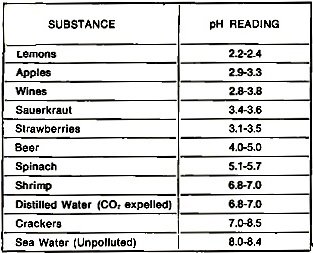
Table 2 - Representative pH readings of familiar substances.
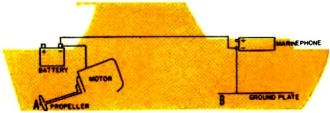
Fig. 2 - A frequent cause of electrolysis corrosion in boats
is the voltage-drop condition that exists between the propeller (A) and the ground
plate (B) due to the long wire run from the negative terminal of the battery to
marine phone.
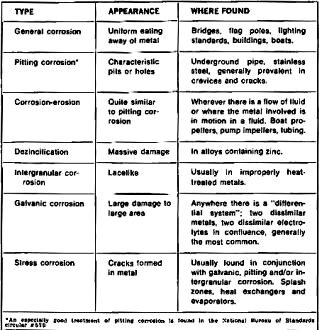
Table 3 - Characteristics of different types of corrosion.

Fig. 3 - Buchler's Chloridometer, an instrument that can be used
for determining the possibility of corrosion by measuring the amount of chloride
present in water.
Rating Galvanic Action
Traditionally, corrosion engineers and scientists while recognizing that an electrolyte,
whether it be a water solution or highly humid air, is necessary for corrosion to
occur, have tended to concern themselves more with the proper choice and treatment
of metals existing in a system to impede or halt corrosion. The base-noble metal
sequence in Table 1 is one of three common listings which have been compiled as
an aid to choosing combinations of metals that will reduce the possibility of galvanic
corrosion. The recommended practice is to either choose identical metals or metals
close together in the base-noble series if they are to be in physical contact with
each other while exposed to a common electrolyte. The farther apart two metals are
from each other in this series, the higher the potential difference between the
two and, therefore, the greater the possibility of corrosion. The same philosophy
applies to both the activity and galvanic series (Table 1). In each of these three
charts, the anodic metal - the one which will suffer the structural corrosion damage
- is at the top, the cathodic metal. The one which will not be affected by the corrosive
process - is at the bottom. The farther apart any two metals are in any of these
series, the stronger the galvanic cell.
The galvanic series is probably the most used series in considering the electronics
of corrosion. This particular galvanic series was predicated on a sea-water electrolyte
and using a silver/silver-chloride reference electrode. Just as in assessing antenna-gain
figures, one must know exactly what the reference unit is. Readings based on a calomel
electrode or a metal electrode would give entirely different readings than those
listed for the galvanic series in Table 1. Fortunately, the silver/silver-chloride
electrode is gradually becoming the standard. As mentioned above, the metal at the
top of the scale will become the anode, the metal at the bottom the cathode. In
the preceding zinc-copper explanation, for instance, the zinc was anodic, the copper
cathodic. Similarly, copper would become the anode when coupled to nickel in a two-
metal, electrolytic, physically connected system. Measurements performed by the
author using the Simpson 313 v.o.m. with a silver/silver-chloride electrode yielded
different values. The reason for the variance was that the author's sea-water samples
came from the highly polluted New York port area while the readings for the galvanic
series in Table 1 were based on relatively unpolluted sea water. In addition, the
New York port area waters have a certain percentage of fresh water introduced by
the Hudson River and other tributaries. The sea water used for the galvanic series
in Table 1 is relatively unpolluted and has no meaningful fresh water content. Thus,
we see that a difference in electrolyte will make a difference in the rate of corrosion.
Indeed, the entire study of corrosion might be said to be a study in differences:
differences in metal, in electrolyte, in - as we will see later - oxygen concentration
in the electrolyte, even differences in size between the anode and cathode involved.
In other words, the corrosion system is a differential system.
Corrosion vs. Electrolyte pH
Just as there are instruments to read the differences in electronic concentration,
i.e., potential (the voltmeter) and to read the flow of electrons (ammeter) , so
also is there a meter to read the active concentration of ionized hydrogen atoms
in a water solution. This is the pH meter and might be regarded as a high-impedance
voltmeter used in conjunction with a special permeable glass probe and a reference
half cell. While the basic function of a pH meter is to determine the active acidity
or alkalinity of a solution, most have an e.m.f. position to read voltages (instruments
without the e.m.f. position may be used to read voltages by substituting 0.06 volt
per pH unit) . Probably the handiest pH meter for the electronics technician would
be the unit made by Analytical Measurements (Chatham, New Jersey). This instrument
(Fig. 1) is portable and battery-powered which permits it being taken "on the scene"
which, in most cases, is a virtual necessity.
The pH meter differs from the voltmeter or ammeter in that it reads "backwards."
While the voltmeter will read higher on the scale when the voltage difference present
is relatively higher, and the ammeter will give a higher indication when the current
is high, the pH meter reads inversely, i.e., the lower the ionized-hydrogen activity,
the higher the pH reading; the higher the ionized-hydrogen activity, the lower the
pH reading. As mentioned above, the pH meter's basic function is the determination
of the active acidity or alkalinity of a solution. Analytical chemists decided that
the more acid a solution the lower the reading, the more alkaline the solution the
higher the reading. The center point of the 0-14 scale -7- was assigned to carbon
dioxide-free distilled water. Some representative pH readings of familiar substances
are given in Table 2.
In practical terms, the electronics technician embarking on corrosion control
will be concerned with only three electrolytes: sea-water, fresh-water, and high-humidity
atmosphere. Comparatively, unpolluted sea water has a pH reading from 8.3 to 8.7
on the 0-14-pH scale. Polluted sea water, such as found in the New York port area,
the Houston ship channel, and other areas in which industrial wastes abound, will
read from 6.0 to 6.6 or sometimes lower. The conclusion: pollution is acid. It follows
that the greater the pollution, whether in an air or water system or interface between
the two, the higher the corrosion possibility. For example, a ship or pleasure boat
with a galvanic arrangement (a metallic-differential system) of a bronze propeller
and a steel rudder and moored or operating in the relatively alkaline environment
of the open sea will not have anywhere the corrosion problem as the same ship moored
in, say, Brooklyn's highly polluted Newtown Creek. In other words, a change in the
electrolytic ecology in which the two dissimilar metals in the galvanic arrangement
find themselves is far more insidious and influential than is usually realized.
This influence of pollution also holds true of corrosion in an air environment.
Here, air with a relatively high percentage of humidity serves as the electrolyte.
The damper climates such as experienced in the Gulf of Mexico and Florida areas,
the Washington-Oregon area, certain portions of the Great Lakes, and, in fact, most
seaboard areas will provide ideal surroundings for corrosion. Couple this ideal
corrosion environment with air pollution and corrosion becomes a major economic
factor. Indeed, some statistics state that one-fifth of the world's annual production
of iron and steel is lost to corrosion. Table 3 lists the different types of corrosion;
their characteristics and where generally found.
Electrolysis Corrosion
A close relative to galvanic corrosion is the so-called electrolysis corrosion
or "stray-current" corrosion. This type arises when the voltage difference in a
corrosion system is not self-generated but is impressed externally. With the advent
of electronic equipment on boats, this kind of corrosion problem assumed increasing
importance. There are two basic areas here and they are both electrical in nature:
externally impressed voltages due to voltage drops in the boat's electrical system,
and cross grounding. Owing to the increasing prevalence of grounding the negative
side of the vessel's electrical system, this latter type is not encountered too
often except in older commercial vessels. The author has several case histories
in which propellers and other underwater metal boat parts have been destroyed in
a matter of weeks due to cross grounding. It is in this area of electrolysis corrosion
that the electronics technician is most usually involved ("I didn't have this corrosion
until you installed the radio!") and generally the villain of the piece is a voltage
drop. Nine times out of ten, a voltage-drop condition can be eliminated by using
the largest practicable size wire for power leads and by bonding all metal parts
of the boat to a common ground strap. This could be a length of strip copper laid
along the keelson with heavy wire or braid going from it to the various metals on
the boat. One common voltage-drop condition involving the marine phone ground-plate
is seen in Fig. 2. The best instrument for detecting voltage drops is one of the
new breed of high-impedance input, battery-operated e.o.m.'s with extra-long clip
leads. The check is made from each metal part to every other metal part and comparing
the power-on with the power-off reading. On land, another pair of leads - even longer
- with 12" to 18" spikes as the probes can be used for determining voltage drops
on buried pipe systems. Gas company officials claim severe corrosion damage to their
natural gas pipelines in the vicinity of electric train tracks despite the use of
high-efficiency magnesium sacrificial anodes.
Local-Cell Corrosion
The two types of corrosion so far discussed have been based on physically obvious
electrical circuits: two metals, a common electrolyte, a complete circuit. But what
causes the corrosion in, say, a metal post with absolutely no connection or relationship
to any other mass of metal? This kind of damage is clue to local-cell action. Just
as local action in a lead-acid storage cell, because of impurities in the electrodes,
can cause sulphation of the plates or the same local cell action can cause the disintegration
of the zinc casing of a dry cell, so also does this phenomenon cause the corrosion
damage in seemingly independent metal structures. One of the most familiar samples
of local cell corrosion is seen by the average person on automobile body parts such
as that shown in the photo on page 42. There are many reasons why these small local
cells are formed on the surface of a piece of metal: impurities in the metal itself,
orientation of the grains in the metal structure, imperfections on the surface,
stresses placed upon the metal when it is used in construction, imperfect homogeneity
in the manufacturing process and others. But in examining local-cell corrosion we
find the identical action taking place that occurred in galvanic corrosion; there
will be many minute anodic and cathodic areas. Each anode and cathode will, obviously,
be physically connected and will also be exposed to a common electrolyte.
Oxygen-Differential Corrosion
In local-cell corrosion, however, the appearance of the damage is different from
that caused by galvanic or electrolysis (externally impressed voltage) corrosion.
It has a characteristically pitted appearance. This pitting process can, however,
multiply and progress until the entire surface is corroded. This is seen in the
familiar "rust" on iron and steel. It is in the area of pitting corrosion that often
there is a merging into yet another form of corrosion: the oxygen-differential system
or the creation of a galvanic cell composed of the same metal in physical contact
but exposed to two different - but connected - electrolytes. The differences in
the electrolytes will be a difference in oxygen concentration.
The oxygen-differential system can be found where one part of a metal structure
is shielded or protected from the air or water while other parts of the same metal
are exposed to the electrolyte. Painting part of a structure and leaving part unpainted
is an invitation to corrosion. Barnacles on boat underwater metal parts will create
a situation where the metal directly underneath the barnacle will be exposed to
an electrolyte having less oxygen than the electrolyte in contact with the adjacent
metal. A simple thing such as the buildup of sand around the base of a metal piling
or post or even sand drifting onto part of a metallic structure can give rise to
the oxygen- differential corrosion system. One very unfortunate and, one might say
"treacherous," example of this type of corrosion may possibly take place under the
zinc sacrificial anodes installed to prevent corrosion. As the zinc corrodes in
its protective action, it will gradually leave a space between itself and the metal
to which it is attached. The electrolyte (water) in this space will have less oxygen
present than water in contact with the adjacent "protected" metal and oxygen-differential
corrosion may take place. This condition may merge into one in which the zinc anode
eventually becomes insulated from the metal part which it is supposed to protect
and galvanic corrosion can then take place between the no-longer-protected metal
and some other metal which is cathodic to it.
Oxygen, then, is another prerequisite to the corrosion process and as can be
expected, we find massive corrosion damage in the so-called "splash zone" or interface
between the air and water environments. The electrical activity of a corrosion system
will be at its highest where wetting and drying, i.e., alternate exposure to the
electrolyte and to the increased oxygen in the air takes place. If we add chloride
(the basic element that differentiates sea water from fresh water) to this system,
we find extensive corrosion damage. The emergent container - ship industry is haunted
by just this type of a problem. Containers, or rather more precisely, the fittings
that are used for moving these containers, may fail long before their projected
life expectancy. An inspection of metal structures along the waterfront of a port
city such as New York will also bear this out. Fig. 3 is a photograph of Buchler's
"Chloridometer," an instrument that can be used for determining the possibility
of corrosion by measuring the amount of chloride present in the water sample.
Anti-Corrosion Techniques
Probably the most common way to combat the ravages of the corrosion process is
by using sacrificial anodes. These are usually of zinc or zinc alloy and are interposed
between the anode and cathode of the galvanic corrosion arrangement and connected
to the anode. In effect, we are substituting a more efficient "battery" component
- the zinc anode -having a higher anodic capability which will dominate the prior
anode and thus protect it.
The true electronic means of protection, however, is the impressed-current approach.
This might be viewed as making an asset of a liability. We have seen that corrosion
can result from impressing an external current on the system (stray-current corrosion).
If we were to use the same concept, but reverse the polarity of the voltage generated
by a galvanic arrangement, we would find that the corrosion process would be effectively
halted. If, for instance, there were a 0.3-volt difference between a bronze propeller
and a steel rudder and these two metals were connected externally by metallic continuity
or, as is often the case, through bilge water we would have the ideal arrangement
for corrosion damage to the anodic rudder ( the bronze propeller being the cathode).
A 0.3 voltage of the opposite polarity impressed across the steel-bronze system
( the physical external connection would first have to be broken) should effectively
prevent corrosion damage to the rudder. The next logical step would be to bond all
the vessel's underwater metal parts together to form a common cathode and then introduce
a balance voltage of sufficient strength via an independent electrical anode so
that the galvanic action would be effectively balanced out. In effect, this system
weakens the anode-cathode relationship to a degree and can be done during the construction
of the boat by using identical metals or metals close to each other in the galvanic
series. This is the basic theory behind the impressed- current or "cathodic" protection
systems. I
t must be stressed that this form of electronic protection will not apply when
stray-current corrosion (voltage drops or cross groundings) or the so-called cavitation
or water-flow corrosion are involved. It is also of doubtful value in oxygen-differential
or area-differential corrosion systems. Even its forte, the halting of galvanic
corrosion, can sometimes be useless because of the difficulty of applying it. In
a case where steel nails are used to install aluminum siding, and there exists the
galvanic reaction between the steel and the aluminum (intense corrosion would be
seen in the aluminum in the vicinity of the nails), there would be no advantage
in attempting impressed current protection methods. The proper practice, of course,
would be to use aluminum nails as a first choice with galvanized-steel nails as
a second. Similarly - although impressed-current philosophy could be made to work
- the corrosion potential between an aluminum lightning rod and a copper grounding
wire would most sensibly be eliminated by using a copper lightning rod or aluminum
grounding wire. In marine work, a like case is seen in marine antennas constructed
of aluminum tubing being fed by a copper transmission line. The new fiber glass
whips with imbedded copper-wire elements have done much to eliminate this common
corrosion problem.
This is not to say that the impressed-current systems are not good. Far from
it. Besides their obvious use in the marine area (oil tank ships with this system
can be constructed with lighter scantlings - on the order of 5-10% - which means
construction costs are less), the protection of pipeline systems can be accomplished
by connecting the pipe to a negative d.c. source and the positive d.c. to electrodes
buried at proper intervals in the ground. The current flow in such a system is from
0.3 to 0.5 milliampere per square foot of pipe, and the voltage will range from
1.5 to 30 volts depending on the resistance of the system.
The impressed-current systems used on boats and ships are all basically the same.
They consist of three elements: a reference electrode, a control unit, and the anode
which introduces the proper current and voltage against the underwater metal parts
to be protected. The reference electrode monitors the amount of protection given
to the boat's underwater metal parts (bonded together to form a common cathode)
and in turn the control unit compares this reference voltage produced by the reference
electrode to the pre-set voltage of the control unit. The output of the controller
is applied to the anode. Since the corrective current is externally ap- plied and
because deterioration of this anode would serve no useful purpose, it is usually
made of one of the metals of the platinum family. Care must be taken that the anode
voltage is not set too high as this may be of doubtful benefit and, in fact, may
cause damage.
In summary, we have progressed from the electronic action which is the basis
for corrosion to an electronic solution to combat this damaging process. Even the
few digressions, such as the discussion of pH, were related to the electro- chemical
phenomenon which is corrosion. Knowledge of such things as pH, chloride content,
and so-on are, of course, valuable to the marine electronics technician. Too often,
for instance, one will find that the malfunction of a transmitter or receiver or
depth-sounder isn't due to something wrong with the unit itself, but to such things
as improper supply voltage, r.f. interference, poor antenna placement, etc. The
same applies to ascertaining the reasons for corrosion. The technician must be prepared
(and this dictates proper instrumentation) to investigate the ecology which surrounds
the vessel on which he has installed or is maintaining electronic equipment. When
he knows the facts of the marine environment, then he'll have intelligent, helpful
answers for the man who says:
"I didn't have this corrosion problem until you installed the radio...!"
*The author is consultant to Marine Surveys Co., Inc., Stillwell & Gladding
(an independent testing laboratory), and Marine Container Equipment Certification
Corp. He recently hosted an industry conference on corrosion problems at Wagner
College's Staten Island (N.Y.) campus.
|














