Module 1 - Introduction to Matter, Energy, and Direct Current |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
Module 1 − Introduction to Matter, Energy, and Direct Current
Pages i, 1−1, 1−11, 1−21, 1−31, 1−41, 1−51, 1−61, 2−1, 2−11, 2−21, 3−1, 3−11, 3−21, 3−31, 3−41, 3−51, 3−61, 3−71, 3−81, 3−91, 3−101, 3−111, 3−121, Appendix I, II, III, IV, V, Index Nickel-Cadmium Cell The nickel-cadmium cell (NiCad) is far superior to the lead-acid cell. In comparison to lead-acid cells, these cells generally require less maintenance throughout their service life in regard to the adding electrolyte or water. The major difference between the nickel-cadmium cell and the lead-acid cell is the material used in the cathode, anode, and electrolyte. In the nickel-cadmium cell the cathode is cadmium hydroxide, the anode is nickel hydroxide, and the electrolyte is potassium hydroxide and water. The nickel-cadmium and lead-acid cells have capacities that are comparable at normal discharge rates, but at high discharge rates the nickel-cadmium cell can deliver a larger amount power. In addition the nickel-cadmium cell can: 1. Be charged in a shorter time, 2. Stay idle longer in any state charge and keep a full charge when stored for a longer period time, and 3. Be charged and discharged any number times without any appreciable damage. Due to their superior capabilities, nickel-cadmium cells are being used extensively in many military applications that require a cell with a high discharge rate. a good example is in the aircraft storage battery. Silver-Zinc Cells The silver-zinc cell is used extensively to power emergency equipment. This type cell is relatively expensive and can be charged and discharged fewer times than other types cells. When compared to the lead-acid or nickel-cadmium cells, these disadvantages are overweighed by the light weight, small size, and good electrical capacity the silver-zinc cell. The silver-zinc cell uses the same electrolyte as the nickel-cadmium cell (potassium hydroxide and water), but the anode and cathode differ from the nickel-cadmium cell. The anode is composed silver oxide and the cathode is made zinc. Silver-Cadmium Cell The silver-cadmium cell is a fairly recent development for use in storage batteries. The silver- cadmium cell combines some the better features the nickel-cadmium and silver-zinc cells. It has more than twice the shelf life the silver-zinc cell and can be recharged many more times. The disadvantages the silver-cadmium cell are high cost and low voltage production. The electrolyte the silver-cadmium cell is potassium hydroxide and water as in the nickel- cadmium and silver-zinc cells. The anode is silver oxide as in the silver-zinc cell and the cathode is cadmium hydroxide as in the NiCad cell. You may notice that different combinations materials are used to form the electrolyte, cathode, and anode different cells. These combinations provide the cells with different qualities for many varied applications. Q21. What are the four basic types secondary (wet) cells? Q22. What are the advantages a NiCad cell over a lead-acid cell?
Q23. What type cell is most commonly used for emergency systems?
Q24. What three cells use the same electrolyte?
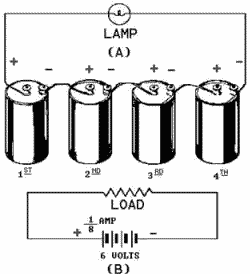
2-11 BATTERIES A battery is a voltage source that uses chemical action to produce a voltage. In many cases the term battery is applied to a single cell, such as the flashlight battery. In the case a flashlight that uses a battery 1.5 volts, the battery is a single cell. The flashlight that is operated by 6 volts uses four cells in a single case and this is a battery composed more than one cell. There are three ways to combine cells to form a battery. COMBINING CELLS In many cases, a battery-powered device may require more electrical energy than one cell can provide. The device may require either a higher voltage or more current, and in some cases both. Under such conditions it is necessary to combine, or interconnect, a sufficient number cells to meet the higher requirements. Cells connected in Series provide a higher voltage, while cells connected in PARALLEL provide a higher current capacity. To provide adequate power when both voltage and current requirements are greater than the capacity one cell, a combination Series-PARALLEL network cells must be used. Series-Connected Cells Assume that a load requires a power supply 6 volts and a current capacity 1/8 ampere. Since a single cell normally supplies a voltage only 1.5 volts, more than one cell is needed. To obtain the higher voltage, the cells are connected in series as shown in figure 2-6.
Figure 2-6. - (A) Pictorial view series-connected cells; (B) Schematic series connection. Figure 2-6 view B is a schematic representation the circuit shown in figure 2-6 view A. The load is shown by the resistance symbol and the battery is indicated by one long and one short line per cell. In a series hookup, the negative electrode (cathode) the first cell is connected to the positive electrode (anode) the second cell, the negative electrode the second to the positive the third, etc.
2-12 The positive electrode the first cell and negative electrode the last cell then serve as the terminals the battery. In this way, the voltage is 1.5 volts for each cell in the series line. There are four cells, so the output terminal voltage is 1.5 x 4, or 6 volts. When connected to the load, 1/8 ampere flows through the load and each cell the battery. This is within the capacity each cell. Therefore, only four series-connected cells are needed to supply this particular load. Caution When connecting cells in series, connect alternate terminals together ( - to +, - to +, etc.) Always have two remaining terminals that are used for connection to the load only. Do not connect the two remaining terminals together as this is a short across the battery and would not only quickly discharge the cells but could cause some types cells to explode. Parallel-Connected Cells In this case, assume an electrical load requires only 1.5 volts, but will require 1/2 ampere current. (Assume that a single cell will supply only 1/8 ampere.) To meet this requirement, the cells are connected in parallel, as shown in figure 2-7 view a and schematically represented in 2-7 view B. In a parallel connection, all positive cell electrodes are connected to one line, and all negative electrodes are connected to the other. No more than one cell is connected between the lines at any one point; so the voltage between the lines is the same as that one cell, or 1.5 volts. However, each cell may contribute its maximum allowable current 1/8 ampere to the line. There are four cells, so the total line current is 1/8 x 4, or 1/2 ampere. In this case four cells in parallel have enough capacity to supply a load requiring 1/2 ampere at 1.5 volts.
Figure 2-7. - (A) Pictorial view parallel-connected cells; (B) Schematic parallel connection.
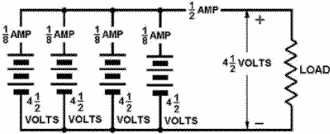
2-13 Series-Parallel-Connected Cells Figure 2-8 depicts a battery network supplying power to a load requiring both a voltage and a current greater than one cell can provide. To provide the required 4.5 volts, groups three 1.5-volt cells are connected in series. To provide the required 1/2 ampere current, four series groups are connected in parallel, each supplying 1/8 ampere current. Figure 2-8. - Schematic series-parallel connected cells. The connections shown have been used to illustrate the various methods combining cells to form a battery. Series, parallel, and series-parallel circuits will be covered in detail in the next chapter, "Direct Current." Some batteries are made from primary cells. When a primary-cell battery is completely discharged, the entire battery must be replaced. Because there is nothing else that can be done to primary cell batteries, the rest the discussion on batteries will be concerned with batteries made secondary cells. Q25. What does the term battery normally refer to? Q26. What are the three ways combining cells, and what is each used for?
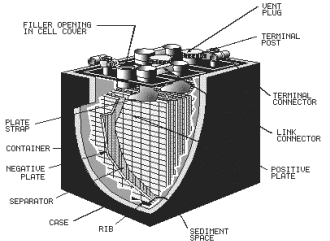
BATTERY CONSTRUCTION Secondary cell batteries are constructed using the various secondary cells already described. The lead-acid battery is one the most common batteries in use today and will be used to explain battery construction. The nickel-cadmium battery is being used with increasing frequency and will also be discussed. Figure 2-9 shows the makeup a lead-acid battery. The container houses the separate cells. Most containers are hard rubber, plastic, or some other material that is resistant to the electrolyte and mechanical shock and will withstand extreme temperatures. The container (battery case) is vented through vent plugs to allow the gases that form within the cells to escape. The plates in the battery are the cathodes and anodes that were discussed earlier. In figure 2-10 the negative plate group is the cathode the individual cells and the positive plate group is the anode. As shown in the figure, the plates are interlaced with a terminal attached to each plate group. The terminals the individual cells are connected together by link connectors as shown in figure 2-9. The cells are connected in series in the battery and the
2-14 positive terminal one end cell becomes the positive terminal the battery. The negative terminal the opposite end cell becomes the negative terminal the battery. Figure 2-9. - Lead-acid battery construction.
Figure 2-10. - Lead-acid battery plate arrangement. The terminals a lead-acid battery are usually identified from one another by their size and markings. The positive terminal, marked (+) is sometimes colored red and is physically larger than the negative terminal, marked ( - ).
2-15 The individual cells the lead-acid battery are not replaceable, so in the event one cell fails the battery must be replaced. The nickel-cadmium battery is similar in construction to the lead-acid battery with the exception that it has individual cells which can be replaced. The cell the NiCad battery is shown in figure 2-11.
Figure 2-11. - Nickel-cadmium cell. The construction secondary cell batteries is so similar, that it is difficult to distinguish the type battery by simply looking at it. The type battery must be known to properly check or recharge the battery. Each battery should have a nameplate that gives a description its type and electrical characteristics. Q27. Other than the type cell used, what is the major difference between the construction the lead- acid and NiCad battery? Q28. How is the type battery most easily determined? BATTERY Maintenance The following information concerns the maintenance secondary-cell batteries and is a general nature. You must check the appropriate technical manuals for the specific type battery prior to performing maintenance on any battery. Specific Gravity For a battery to work properly, its electrolyte (water plus active ingredient) must contain a certain amount active ingredient. Since the active ingredient is dissolved in the water, the amount active ingredient cannot be measured directly. An indirect way to determine whether or not the electrolyte contains the proper amount active ingredient is to measure the electrolyte's specific gravity. Specific gravity is the ratio the weight a certain amount a given substance compared to the weight the same amount pure water. The specific gravity pure water is 1.0. Any substance that floats has a specific gravity less than 1.0. Any substance that sinks has a specific gravity greater than 1.0.
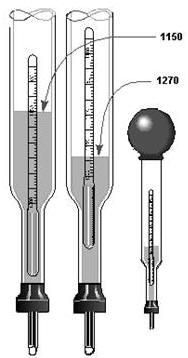
2-16 The active ingredient in electrolyte (sulfuric acid, potassium hydroxide, etc.) is heavier than water. Therefore, the electrolyte has a specific gravity greater than 1.0. The acceptable range specific gravity for a given battery is provided by the battery's manufacturer. To measure a battery's specific gravity, use an instrument called a HYDROMETER. The Hydrometer A hydrometer, shown in figure 2-12, is a glass syringe with a float inside it. The float is a hollow glass tube sealed at both ends and weighted at the bottom end, with a scale calibrated in specific gravity marked on its side. To test an electrolyte, draw it into the hydrometer using the suction bulb. Draw enough electrolyte into the hydrometer to make the float rise. Do not draw in so much electrolyte that the float rises into the suction bulb. The float will rise to a point determined by the specific gravity the electrolyte. If the electrolyte contains a large amount active ingredient, its specific gravity will be relatively high. The float will rise higher than it would if the electrolyte contained only a small amount active ingredient.
Figure 2-12. - Hydrometer. To read the hydrometer, hold it in a vertical position and read the scale at the point that surface the electrolyte touches the float. Refer to the manufacturer's technical manual to determine whether or not the battery's specific gravity is within specifications. Note: Hydrometers should be flushed with fresh water after each use to prevent inaccurate readings. Storage battery hydrometers must not be used for any other purpose. Q29. What is the purpose the hydrometer?
2-17 Q30. Which electrolyte has more active ingredient? Electrolyte A, specific gravity 1.015? Electrolyte B, specific gravity 1.125? Other Maintenance The routine maintenance a battery is very simple. Terminals should be checked periodically for cleanliness and good electrical connection. The battery case should be inspected for cleanliness and evidence damage. The level electrolyte should be checked and if the electrolyte is low, distilled water should be added to bring the electrolyte to the proper level. Maintenance procedures for batteries are normally determined by higher authority and each command will have detailed procedures for battery care and maintenance. Safety Precautions With Batteries All types batteries should be handled with care: 1. NEVER Short The TERMINALS a BATTERY. 2. CARRYING STRAPS SHOULD BE useD When TRANSPORTING BATTERIES. 3. PROTECTIVE CLOTHING, SUCH AS RUBBER APRON, RUBBER GLOVES, and a FACE SHIELD SHOULD BE WORN When Working WITH BATTERIES. 4. NO SMOKING, ELECTRIC SPARKS, OR OPEN FLAMES SHOULD BE PERMITTED NEAR CHARGING BATTERIES. 5. CARE SHOULD BE TAKEN to PREVENT SPILLING The ELECTROLYTE. In the event electrolyte is splashed or spilled on a surface, such as the floor or table, it should be diluted with large quantities water and cleaned up immediately. If the electrolyte is spilled or splashed on the skin or eyes, IMMEDIATELY flush the skin or eyes with large quantities fresh water for a minimum 15 minutes. If the electrolyte is in the eyes, be sure the upper and lower eyelids are pulled out sufficiently to allow the fresh water to flush under the eyelids. The medical department should be notified as soon as possible and informed the type electrolyte and the location the accident. CAPACITY and RATING BATTERIES The CAPACITY a battery is measured in ampere-hours. The ampere-hour capacity is equal to the product the current in amperes and the time in hours during which the battery will supply this current. The ampere-hour capacity varies inversely with the discharge current. For example, a 400 ampere-hour battery will deliver 400 amperes for 1 hour or 100 amperes for 4 hours. Storage batteries are RATED according to their rate discharge and ampere-hour capacity. Most batteries are rated according to a 20-hour rate discharge. That is, if a fully charged battery is completely discharged during a 20-hour period, it is discharged at the 20-hour rate. Thus, if a battery can deliver 20 amperes continuously for 20 hours, the battery has a rating 20 amperes x 20 hours, or 400 ampere-hours. Therefore, the 20-hour rating is equal to the average current that a battery is capable supplying without interruption for an interval 20 hours. (Note: Aircraft batteries are rated according to a 1-hour rate discharge.)
2-18 All standard batteries deliver 100 percent their available capacity if discharged in 20 hours or more, but they will deliver less than their available capacity if discharged at a faster rate. The faster they discharge, the less ampere-hour capacity they have. The low-voltage limit, as specified by the manufacturer, is the limit beyond which very little useful energy can be obtained from a battery. This low-voltage limit is normally a test used in battery shops to determine the condition a battery. Q31. When should safety precautions pertaining to batteries be observed? Q32. How long should a 200 ampere-hour battery be able to deliver 5 amperes? BATTERY CHARGING It should be remembered that adding the active ingredient to the electrolyte a discharged battery does not recharge the battery. Adding the active ingredient only increases the specific gravity the electrolyte and does not convert the plates back to active material, and so does not bring the battery back to a charged condition. a charging current must be passed through the battery to recharge it. Batteries are usually charged in battery shops. Each shop will have specific charging procedures for the types batteries to be charged. The following discussion will introduce you to the types battery charges. The following types charges may be given to a storage battery, depending upon the condition the battery: 1. Initial charge 2. Normal charge 3. Equalizing charge 4. Floating charge 5. Fast charge Initial Charge When a new battery is shipped dry, the plates are in an uncharged condition. After the electrolyte has been added, it is necessary to charge the battery. This is accomplished by giving the battery a long low- rate initial charge. The charge is given in accordance with the manufacturer's instructions, which are shipped with each battery. If the manufacturer's instructions are not available, reference should be made to the detailed instructions for charging batteries found in current Navy directives. Normal Charge A normal charge is a routine charge that is given in accordance with the nameplate data during the ordinary cycle operation to restore the battery to its charged condition. Equalizing Charge An equalizing charge is a special extended normal charge that is given periodically to batteries as part a maintenance routine. It ensures that all the sulfate is driven from the plates and that all the cells
2-19 are restored to a maximum specific gravity. The equalizing charge is continued until the specific gravity all cells, corrected for temperature, shows no change for a 4-hour period. Floating Charge In a floating charge, the charging rate is determined by the battery voltage rather than by a definite current value. The floating charge is used to keep a battery at full charge while the battery is idle or in light duty. It is sometimes referred to as a trickle charge and is accomplished with low current. Fast Charge A fast charge is used when a battery must be recharged in the shortest possible time. The charge starts at a much higher rate than is normally used for charging. It should be used only in an emergency, as this type charge may be harmful to the battery. Charging Rate Normally, the charging rate Navy storage batteries is given on the battery nameplate. If the available charging equipment does not have the desired charging rates, the nearest available rates should be used. However, the rate should never be so high that violent gassing (explained later in this text) occurs. Charging Time The charge must be continued until the battery is fully charged. Frequent readings specific gravity should be taken during the charge and compared with the reading taken before the battery was placed on charge. Gassing When a battery is being charged, a portion the energy breaks down the water in the electrolyte. Hydrogen is released at the negative plates and oxygen at the positive plates. These gases bubble up through the electrolyte and collect in the air space at the top the cell. If violent gassing occurs when the battery is first placed on charge, the charging rate is too high. If the rate is not too high, steady gassing develops as the charging proceeds, indicating that the battery is nearing a fully charged condition.
Warning A mixture hydrogen and air can be dangerously explosive. No smoking, electric sparks, or open flames should be permitted near charging batteries. Q33. Can a battery be recharged by adding more electrolyte? Q34. If violent gassing occurs during a battery charge, what action should be taken? Summary In this chapter you learned that batteries are widely used as sources direct-current. You were introduced to electrochemical action and the way it works in a cell, the cell itself, the type and parts a cell, and how cells are connected together to form batteries. You learned the construction and maintenance batteries and some the safety precautions in handling and working with batteries.
2-20
|
||||||||||||||||||||||||||||||||||||||||||||||||||