|
July 1954 Popular Science
 [Table of Contents] [Table of Contents]
Wax nostalgic about and learn from the history of early
electronics. See articles from
Popular
Science, published 1872-2021. All copyrights hereby acknowledged.
|
A lot of people think the
modern day solar energy craze is a relatively new phenomenon, but old guys like
me remember back in the early 1970s, during the
Arab Oil Embargo, when we were
promised that massive arrays of photovoltaic (PV) cells would save the world
from its dependence on foreign oil suppliers. At the time, the massive oil
reserves below the ground here in the USA, and in easily accessed offshore
regions, were not known. PV cell manufacturers (small by today's standards) were
popping up around the country. I remember one in particular,
Solarex
Corporation, fairly nearby in Frederick, Maryland (north of D.C.), which
served as a sort of poster child for solar power, went
toes-up due to
underperformance in conversion efficiency and excessive costs. Tragically, we were told by
"experts" that between wind, solar, hydro, and nuclear, that electricity would
be practically free, and many homes and businesses had electric heating
installed in the form of baseboard electric heaters. Now, with closing nuclear
plants after the 3-Mile Island incident and the current government shutting down
gas and oil mining and pushing expensive "renewable" energy sources, people are
getting winter electricity bills over $400/month. This 1954 Popular Science
magazine article reports on some of the very earliest attempts at solar cell
manufacturing.
Sunshine Becomes Electricity
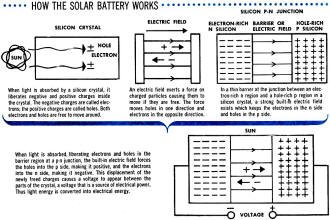
How the Solar Battery Works
When light is absorbed by a silicon crystal, it liberates negative and positive
charges inside the crystal. The negative charges are called electrons; the positive
charges are called holes. Both electrons and holes are free to move around.
An electric field exerts a force on charged particles causing them to move if
they are free. The force moves holes in one direction and electrons in the opposite
direction.
In a thin barrier at the junction between an electron-rich n region and a hole-rich
p region in a silicon crystal, a strong built-in electric field exists which keeps
the electrons in the n side and holes in the p side.
When light is absorbed, liberating electrons and holes in the barrier region
at a p-n junction, the built-in electric field forces the holes into the p side,
making it positive, and the electrons into the n side, making it negative. This
displacement of the newly freed charges causes a voltage to appear between the parts
of the crystal, a voltage that is a source of electrical power. Thus light energy
is converted into electrical energy.
There's something really new under this summer's sun. It's about half the size
of this magazine, and it "taps" sunlight for electricity. It yields enough power
to operate a transmitter, run a little motor or make a phone call.
Directly, instantly and efficiently, it transforms solar energy into electrical
power. When light shines on it, you can use it just as though it were a dry battery;
when the light goes out, it stops. It may never wear out.

Strips of silicon the size of razor blades yield more power than an atomic battery.
On top of a telephone pole, it could be used to convert sunshine into power for
the telephone line. It works on artificial light, too. Recently its inventors turned
a spotlight on one in a dark room and made enough electricity to run a small motor
that turned a toy Ferris wheel.
Credit for developing this astonishingly simple way of turning daylight, which
is free, into electricity, which we usually buy, goes to a physicist, a chemist
and an electrical engineer on the staff of the Bell Telephone Laboratories. The
batteries which they showed to the National Academy of Sciences this spring delivered
millions of times as much power as the atomic battery that was demonstrated a few
weeks earlier (PSM, Apr. '54, p. 112). The makers of the solar battery believe,
moreover, that its efficiency probably can be doubled.
Their discovery grew out of their study of semiconductors of electricity, and
is based on a "p-n junction," which is used in transistors. The "p" stands for positive,
the "n" for negative, and the junction is a barrier between parts of the material
that react oppositely. In this case, the material is silicon -a light, abundant
element that looks metallic and is found in common sand.
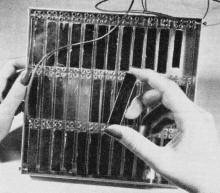
This is One of the First solar batteries ever made. It consists
of strips of specially treated silicon, which are connected together and mounted
in plastic.
First, the silicon is purified until the ratio of non-silicon atoms in it is 1
to 10,000,000. Impurities are then put into it. These create a surplus of electrons
throughout the stuff.

The Three Inventors of the solar battery are (left to right):
G. L. Pearson, a physicist; D. M. Chapin, an electrical engineer; and C. S. Fuller,
a chemist.
The next step is to treat slices of silicon in such a way that, instead of a
surplus of electrons, there will be a scarcity of them on the surface of each slice.
This is done by heating the silicon in a tube containing a different kind of impurity.
The two different impurities cause the silicon to react differently when light strikes
it; its surface becomes positive and its interior negative.
Tiny wires then are attached to the surface, and to the interior of each slice,
and electricity is drawn through those wires whenever the silicon is exposed to
light. By connecting many such slices together, 50 watts of power per square yard
of surface can be drawn from sunlight.
Previous, comparable devices - thermocouples and photoelectric cells - have not
converted more than one percent of light's energy into electricity; this new device
utilizes six percent of the light. This compares favorably with the efficiency of
steam and gasoline engines.
First Use for Telephones and Radios
The scientists expect these remarkable new batteries to be used first for rural
telephone lines and low-power mobile radio apparatus. But many more uses may be
found for them. Conceivably, at least, a roof built of strips of silicon will someday
convert the sunlight shining on it into electricity to run the air-conditioner in
the basement. The cost would be prohibitive now, but prices are man-made figures
that men have often remade. -Volta Torrey.
Posted January 18, 2024
|









