|
February 1944 Popular Science
 [Table of Contents] [Table of Contents]
Wax nostalgic about and learn from the history of early
electronics. See articles from
Popular
Science, published 1872-2021. All copyrights hereby acknowledged.
|
Prior to taking my first
chemistry course while in engineering school, I had experience with using
catalysts in compounds like epoxy adhesives and paints, fiberglass resin, and
body putty for fixing cars. Usually the catalyst part was called a "hardener."
It seemed to me the end result was a solid mass with no apparent separate
leftover component. My freshman semester professor's* claim that, similar to
author Swezey's statement, "A catalyst is a substance which can change the speed
of reaction between two or more other substances, without being permanently
changed or used up," was amazing to me. Where did the catalyst go, then? This "Catalysts ... Secret Agents of Chemistry"
article from a 1944 issue of Popular Science magazine does a nice job of
explaining the process, and gives a few examples of how a catalyst works.
* The same professor taught me an
ideal gas law mnemonic
which goes: "Oh, my gracious goodness me, PV=nRT." It must have worked ;-)
Catalysts ... Secret Agents of Chemistry
 By
Kenneth M. Swezey By
Kenneth M. Swezey
By means of a new catalytic oil-cracking process, American industrial chemists
claim they can now produce a superfuel which is superior to any gasoline heretofore
commercially available. Mix this fuel with ordinary gasoline, and present airplanes
will travel faster or carry greater loads. Use it alone, and the fuel permits the
design of engines tremendously more powerful and faster than those used today.
Once more a catalyst has worked a miracle, and by so doing has again attracted
the attention of the public to one of the most mysterious and fascinating subjects
of modern Chemistry.
In newspapers, schoolbooks, and in these home chemistry articles, we have read
how catalytic processes help make sulphuric and nitric acids, ammonia, dyes, alcohol,
plastics, synthetic rubber. In hundreds of other processes we seldom hear of, some
catalyst is equally vital. Just what is a catalyst, and how does it work?
The answer to the first part of the question is easy, but the second poses a
problem which chemists have never solved satisfactorily. A catalyst is a substance
which can change the speed of reaction between two or more other substances, without
being permanently changed or used up. Jokingly, it has been called a chemical parson
- an agent which can bring about the union of other substances and yet remain unchanged
itself.
Sometimes the speed of reaction can be accelerated by a catalyst to such a tremendous
degree that the catalyst seems to produce the reaction. Take a little mound of dry
baking powder, for instance. As long as this powder remains perfectly dry it might
remain almost indefinitely without decomposition. If you add a few drops of water,
however, the seemingly inert powder immediately froths up with volumes of gas. Plain
water has in this case acted as a catalyst, helping the acid compound and the sodium
bicarbonate to unite, liberating carbon dioxide. The water is not changed by the
reaction.
Water is probably the most universal catalyst. Many chemical reactions apparently
cannot take place without at least a trace of moisture. You can easily demonstrate
a violent and vivid reaction between normally nonreactive materials, initiated by
a single drop of water. Because of the dense, choking fumes produced, it is best
to perform this experiment outdoors. or near an open window.
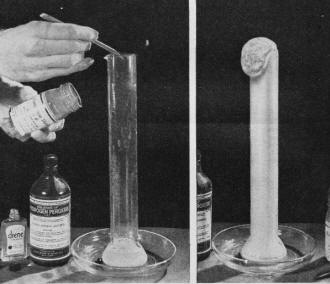
When a catalyst, a few grains of manganese dioxide, is dropped
into a tall beaker holding some hydrogen peroxide, a rapid decomposition of the
peroxide ensues. If shampoo is added, liberated oxygen makes foam.
Powder some dry iodine crystals in a mortar and mix thoroughly with this powder
an equal volume of powdered aluminum first making certain that both substances are
absolutely dry. Place a little mound of the mixture on the center of an asbestos
pad. Now add a drop of water in a depression on top of the mound, and stand back.
Almost instantly, purple-red vapors rise from the heap. In another second, the mound
bursts into beautiful purple-red flame, accompanied by great volumes of smoke.
Berzelius, the great Swedish chemist, devised the term catalytic agent in 1836.
Long before that, however, catalysts had been used. Sulphuric acid, for example,
served as a catalyst in making ether - or "sulphuric ether," as it is sometimes
still called. Ether - or, more properly, ethyl ether - is produced from ethyl alcohol
when two molecules of the alcohol lose one molecule of water. Sulphuric acid helps
remove the water.
To demonstrate this transformation in your home laboratory, pour about 2 ml.
of denatured alcohol into a test tube and carefully add an equal amount of concentrated
sulphuric acid. Pour in the acid very slowly, mixing the solution cautiously with
a glass stirring rod. Now heat the mixture gently over a small alcohol or Bunsen
flame. Keep the tube turned away from you, and do not bring the mixture to a boil.
This experiment is entirely harmless if you follow directions; the cautions are
merely to prevent any spilling or spattering of the strong acid, which might cause
serious burns to yourself or damage to your workbench.
After heating the acid-alcohol mixture for a few seconds, pour it into about
20 ml. of warm water in a beaker. The presence of ether can immediately be detected
by its smell. The sulphuric acid is merely diluted by the water removed from the
alcohol during this reaction. None of the acid is otherwise altered or lost.
A common home chemistry experiment shows how manganese dioxide serves as a catalyst
to release oxygen from hydrogen peroxide. Here is a method of demonstrating this
catalytic process even more vividly. Pour a little hydrogen peroxide into a tall
glass or beaker and mix with this a few drops of some soapless shampoo. Now drop
in a few grains of powdered manganese dioxide. Instantly, oxygen released from the
decomposing peroxide starts making foam, and if enough solution is used, the foam
will finally over-flow the glass.
Perhaps without realizing it, you use a catalyst every time you mix a drier into
paint. The drying of linseed oil is not merely evaporation, such as occurs when
turpentine dries. It consists of a hardening of the oil into a tough film, caused
by oxidation of the oil. Driers, which usually consist of borates or resinates of
manganese, cobalt, or other metals, help hasten this oxidation.

Water is often a catalyst. Here a single drop, added to powdered
iodine crystals and powdered aluminum, is sufficient to make the mixture burst into
colorful flames. Do this in a ventilated room.

Metallic catalysis can be demonstrated with a spiral of bare
copper wire heated to a dull redness. Placed in the vapor of warm methyl alcohol,
it causes an alcohol-oxygen reaction, indicated by an odor of formaldehyde.
You can easily make some manganese borate, a typical drier, and find out for
yourself how it works. Dissolve a little borax in one portion of water, and a little
manganese sulphate or chloride in another portion. If you now mix the two solutions,
a flesh-colored precipitate of manganese borate will form. This substance is recovered
by filtering off the liquid. While still on the filter paper, it is washed with
water to remove impurities and is then dried.
Now paint two strips of cloth with linseed oil-one with plain oil, and the other
with oil in which a few grains of the manganese borate have been mixed. After exposure
to the air for a day, the cloth coated with linseed oil containing the drier becomes
dry and stiff; while the other cloth, coated with plain linseed oil, is still limp
and wet.
The catalysts thus far mentioned have been powders or liquids which have been
mixed with the substances between which they have promoted a reaction. In another
type of catalyst the reaction occurs when gases or other substances are caused to
unite by being strongly adsorbed together on the surface of some metal, such as
spongy platinum, platinum black, copper, or finely divided iron or nickel.
The flameless, sparkless stove lighters of the past, as well as modern flameless
cigarette lighters, depend for their operation on catalysts of this type. In these
lighters, illuminating gas in the first case, and alcohol vapor in the second, oxidizes
vigorously upon touching the surface of a platinum or palladium catalyst. The intensity
of the reaction causes the catalyst to heat up to redness.
When lighters of this kind fail to work properly, the trouble often springs from
a "poisoned" catalyst, a condition produced by an accumulation of dirt or soot on
the surface of the catalytic metal. (Because an extremely small quantity of an impurity
can affect the power of a catalyst, chemists sometimes use this fact to make sensitive
analyses. For example, very minute concentrations of cyanide can be detected by
their effect in reducing the catalytic activity of platinum.) If your flameless
cigarette lighter has a catalytic element coated with soot, you can usually renew
it by holding the unit for a few seconds in the tip of a blow-pipe flame, as shown
in the photo on page 190.
Smooth copper screening is used as a surface catalyst in changing methyl alcohol
into formaldehyde by a process of slow oxidation. This reaction can easily be demonstrated
with the aid of a small spiral of copper wire. Procure about eight inches of No.
14 or 18 bare copper wire and form the end into a spiral by winding it around a
pencil.
Gently warm a little methyl alcohol in a test tube. Heat the copper spiral in
a Bunsen flame to dull redness, and then plunge this coil into the vapor in the
test tube. Any coating of black copper oxide which may be on the wire immediately
vanishes, and the pungent odor of formaldehyde issues from the tube. The hot copper
causes the alcohol vapors to unite with oxygen from the air and from its own oxide.
Fame awaits whoever solves the mystery of why catalysts produce their results.
Posted
|














