Electric Power Directly from Gas
|
|
Once a major manufacturer of both primary (one-time use) and secondary (rechargeable) batteries, the National Carbon Company changed names many times through acquisitions and mergers until its current incarnation as GrafTech International. National Carbon Co. appeared frequently in electronics magazines during the World War II era for their innovations in portable power supplies. They are credited for developing the world's first "D" size dry cell battery. This advertisement in a 1958 issue of Popular Electronics heralded the company's introduction of what was essentially what we call a hydrogen fuel cell. See the more extensive "Recent Developments in Battery Design" article in the July 1959 issue of Popular Electronics, which included this information. Electric Power Directly from Gas
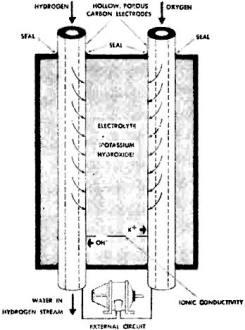
The secret of the cell's success lies in chemically treated, hollow, porous carbon electrodes, through which the gases enter the cell and which conduct the electricity produced by the electrochemical reaction. Hydrogen and oxygen enter the cell through the electrodes and diffuse through the porous carbon to the surface, where they come into contact with the electrolyte - potassium hydroxide. At the hydrogen electrode, the reaction with the electrolyte produces water and releases electrons which enter the electrical circuit. These electrons flow through the external circuit and return to the cell at the oxygen electrode where, in the electrochemical reaction of the oxygen and the electrolyte, the electrons are accepted. Ionic conductivity through the electrolyte completes the circuit.
Simplified drawing to the right illustrates basic operation of the fuel cell. See story for details. The cell has about 1 volt potential and the current available depends upon its size, so by varying the number and size of cells, many combinations of current and voltage may be obtained. Basically, the cell is most efficient for high-current, low-voltage use. Photo at top of page shows Signal Corpsmen testing "Silent Sentry" radar powered by the new cell. The equipment is capable of spotting a single person a half-mile away in total darkness. The battery of cells (not visible) supplies power at 28 volts.
Posted October 4, 2019 |
|