Can Dry Cells Be Recharged?
|
|
Radio Shack Archer Model No. 23-120A Battery Charger
As one who has been dealing with using and recharging batteries - both individual cells and wired packs - for about five decades, I have used many varieties of chargers and battery chemistries. As you probably know, there are two basic types of cells: primary and secondary. Based on their construction and chemistry, the former are not designed to be recharged while the latter are. Primary cells include zinc-carbon and standard alkaline. Secondary cells include nickel-cadmium (NiCd), nickel-metal-hydride (NiMH), and lithium polymer (Li-Po). Rechargeable batteries can have their discharge cycle reversed by running an externally supplied current through the cell in the opposite direction. Of course there are optimal conditions by which that current must be fed in order to preserve the cell's properties (peak voltage, charge storing capacity, discharge current, internal resistance, current leakage, etc.) and lifetime. There have been many occasions where I put primary batteries in a charger for a few hours in order to squeeze a little more use out of them. In all the years, I have never had one explode, catch fire, overheat, leak chemicals, or any scary thing. I used the same set of standard alkaline "D" cells in flashlights for years. In fact, I still have a 1970s vintage Radio Shack dry cell charger designed to work with carbon batteries. In the 1990s and even early 2000s, there were many high-current consumer products like cassette and CD music players and digital cameras that went through a set of batteries in no time. Using rechargeable alkaline, NiCad (aka NiCd), or NiMH cells didn't help much because their nominal voltages are around 1.20-1.25 volts (vs. 1.5 volts) so they started out at a voltage near to the bottom of the operational voltage and didn't last very long. It wasn't until fairly recently that products started being designed to work at the lower voltage levels of secondary cells. Can Dry Cells Be Recharged? 
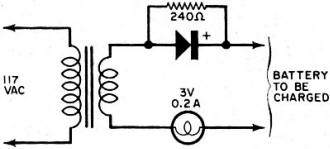
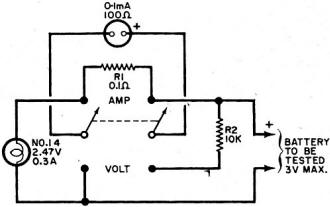
By Fred Shunaman You Can Get up to 20 to 30 Times More Life out of Your Battery Way back in the days when I was powering my peanut tube (Western Electric 215A to the historians) with a No.6 dry cell, recharging a dry cell was no problem. We simply took the cardboard case off the cell, punched a few nail holes in the zinc, and dunked it in a fruit jar filled with a solution of water and sal ammoniac. The thinking was that there was still some zinc in the cell and that it probably lacked moisture. The cell's life was increased tremendously - it ran until big holes appeared in the zinc. But charging a dry cell electrically? That was something else again. There were persistent rumors that someone had done it, but the manufacturers' instructions were explicit. "Dry cells are not designed to be recharged," they said. So the matter rested in indecision until a.c. sets came along and we forgot all about it - for a while. Fig. 1 - Resistor across rectifier allows a small amount of reverse current to flow while charging a battery. A small capacitor can be used instead of the resistor. Fig. 2 - Test jig made it possible to check battery voltage and current while the battery was discharged through the lamp. Open-circuit battery voltage can be measured by partially unscrewing the lamp. Shortly after the transistor radio appeared on the market, dry battery charging began to stage a comeback. Besides the transistor sets, children's toys were using large quantities of these cells - nothing beats a stalled toy electric motor for running down a battery. A few chargers were offered to the public, usually through highly pictorial Sunday-supplement advertising. I picked up a charger and tried it out on my collection of dry cells, some of which were nearly new. Running the cells in a flashlight until the light dimmed and turned yellow, then recharging the cells overnight or a little longer, I came to the conclusion that the life of a dry cell could be about doubled, with a large number of recharges. Shortly afterward, I read an article by a British author, Ralph W. Hallows, in which he claimed excellent results with the help of a technique borrowed from electroplating. He used a small amount of a.c. along with the d.c. charging current-about 10% reverse current. (Half of the time, the a.c. looks like a reverse charging current.) "This technique," he said, "deposited the zinc smoothly and evenly on the inside of the can, instead of in spongy clumps." Commercial chargers using this principle (Fig. 1) were in use in Europe, according to Hallows, and he had recharged flashlight cells repeatedly for more than 18 months of service. I made a note to try the system, but just didn't get around to it. All this was more than a decade ago. But the growing popularity and flood of transistor radios, phonographs, tape recorders and the other battery-operated devices has made the subject topical again. A number of charges are now on the market and available in department stores, hardware stores, hobby stores, electronics parts stores and in variety stores. They are available by mail order and as premium gifts. At the present rate of market penetration, there will be more battery chargers in this country than there are bathtubs, or refrigerators, within the next year or two. There are good chargers and there are bad chargers, and price is not always the best guide to quality. Coming Next Month Plans for combination battery tester and charger that lets you control and monitor charging current and voltage as well as perform tests under desired loading conditions! Project designed and built by Harley H. Stover. I obtained an inexpensive charger, one that would handle four dry cells at a time. In spite of its low price, the appearance of the equipment inspired confidence - it was well constructed out of plastic, and looked as if it were intended for use. An ingenious shorting device made it equally easy to charge one or four cells, and the contacts immediately suggested the right way to insert the batteries. An interlock disconnected the 117-volt power line whenever the unit was open, for safety. I was almost ready to prove or disprove to myself some of the claims about charging dry batteries. Reading up a little on the subject, I found that-among other things - a 1 1/2-volt cell should not be allowed to drop below 1 volt. So a little test jig (circuit shown in Fig. 2) was constructed to make it easy to check the condition of the battery at any time. Either the voltage under load, or the current, could be determined at the flip of a switch. Also, unscrewing the lamp made it possible to determine open-circuit voltage. I bought four RCA VS 036 cells: two for putting through the charger as many times as possible, and two to act as controls by using them without the benefit of being recharged. Putting a pair into the jig, and running them down, and then putting the other pair into the jig and doing the same thing, I learned an interesting fact: just how good a flashlight cell is. Both pairs discharged down to 1.8 volts, or 0.9 volt per cell, and kept the lamp lit for an identical time (3 hours and 40 minutes). "Removed from service," each cell showed 1.1 volt on open circuit. Fig. 3 - This inexpensive. commercially available unit, with modification, was used to charge the batteries. An interlock similar to the type used on TV sets prevents the a.c. line voltage from getting at any of the exposed battery connections while the lid is up. Set 1 was placed in the commercially available charger (Fig. 3) and charged in accordance with instructions that came with the charger. The batteries were then returned to service, and "ran" nearly as long as the first time. (Incidentally, I modified the charger to supply reverse current. It seemed easier to install a capacitor than a resistor in the space I had, so I put a 0.08-μF paper capacitor across both the "lamp-resistor" and the diode, as shown in the dashed lines in Fig. 4. Unfortunately, no comparison was made of charging batteries with and without this capacitor.) The first three charges added an additional 9 hours to the original almost 4 hours of life. Now I learned a second interesting thing about dry cells: the ability of a run-down dry cell to recuperate. Remembering that batteries tended to recover somewhat after discharge, after two days of rest I put my control cells (set 2) back into the test jig. They again lit the lamp to full brilliancy, with a loaded voltage of 1.45 each (as against 1.5 when they were absolutely new) and "ran" for nearly three hours (170 minutes) on the second discharge. With two-day rests between each discharge, set 2 went through two additional discharges, one for 100 minutes, and the other for 90 minutes. After two more days of rest, they measured 1.5 volts open circuit, but only 0.8 volt loaded, and were considered to be at the end of their useful life. They had worked a flashlight lamp for an approximate total of 9 1/2 hours. The control cells were, for practical purposes, of no further use, but I thought it might be interesting to see what would happen if I tried to charge them up. After a 36-hour charge, they showed 3.7 volts (1.85 each) on open circuit, and 3.4 volts when loaded down with 350 mA of current through the lamp. The lamp stayed on for 3 hours and 40 minutes (surprisingly, the same as when the batteries were new) before the batteries dropped to 1.8 volts (under load); the light had not as yet started to dim appreciably. So I continued to put them on a regular charge-discharge cycle and was able to drain another 34 hours and 35 minutes out of those "used up" cells, for a total of 44 hours, 15 minutes. So it appears that the story that, "You can't charge a run-down cell," needs modification. However, there seem to be a number of variables as to how much "second-life" batteries have. Much depends upon their age, state of charge while standing around and not in use, and how the batteries were used. Batteries that have been run down in a normal manner and then put on charge soon after do take and hold a charge. On the other hand, I tested two pairs of cells from flashlights as soon as they ceased to give enough light, after several months of intermittent service; neither pair would light a lamp more than momentarily after being taken off charge. Meanwhile, set 1 was going through cycle after cycle of charge and discharge, dropping down to 180 minutes of life per charge, from the original 220. After the first few discharges, battery life per charge continued to decline more slowly and leveled off at about 170 minutes. For more than another dozen charges, it maintained this level, ±5 to 10 minutes. After another two dozen charge and discharge cycles, the batteries dropped to less than 120 minutes per discharge. Around the 30th cycle, no more than 60 minutes at a time of useful life was obtained. See Fig. 5. Fig. 4 - The lamp in the charging circuit serves as a current limiter. The charger was modified by adding a 0.08-μF capacitor to provide a small amount of reverse charging current. Unfortunately tests were not made to see if the addition of this capacitor made any difference in extending battery life, or in improving charging action. Fig. 5 - Two 1.5-volt "D" cells placed in series were discharged to about 1.8 volts (0.9 volts each cell) and then charged. Graph shows number of hours it took for batteries to drain down to 1.8 volts after each charge. About 2 1/2 hours of life per charge was realized between the 8th and the 21st charge. Tests were stopped when less than one hour of continuous battery operation could be expected. Shelf life was not determined. I abandoned charging after the 31st round. The pair of cells had worked a flashlight lamp for about 68 hours, or roughly seven times the "normal" expected life. They still had potential life in them, but I didn't think it was worthwhile to charge a battery that couldn't produce more than a single hour of illumination. My experiments were not quite up to laboratory precision. Other work interfered with the regularity of the charging and discharging cycles. It wasn't possible for me to always stop the discharge cycle at exactly 1.8 volts for the two cells. But the exceptions were indeed rule-provers and showed that the cells, under- or over-discharged, tended to return to their proper place on the "hours vs. discharges" curve. Of course, in actual use, the start and stop of a charge-discharge cycle can also be expected to take place at other than ideal times. Authorities say that recharged cells have a shorter shelf life than new ones. It is likely that if the cells I used had been put in a flashlight and used intermittently for about a month until the voltage dropped to the recharge point, the cell life would have been much shorter in terms of total milliampere-hours. However, the experimental conditions more closely resembled certain industrial and law-enforcement applications, where flashligh ts are used every night and re-turned for charging every morning. While I confined my experiments to ordinary carbon-zinc D-cells (and kept the charger tied up three months doing only that), I did get a chance to try a charge or two on some of the smaller cells. Results appeared similar. One set of AA's, in particular, was taken out of an illuminated probe, where they no longer lit the lamp. After being recharged, they brought the lamp up to full brightness, and remained useful for about two weeks. I did not try to charge alkaline cells, but there is sufficient reason to believe that these cells can also be recharged. Mercury batteries are another story. In theory, they are highly chargeable, but in practice they come in such a variety of sizes and voltages that they can be difficult to handle. I brought leads out from my charger to one small cell. Two hours later I found the case empty. I have not yet found the top or the contents of the cell. Could be that the battery blew up because of an excessive charging rate. Fig. 6 - Hearing-aid battery adapter has movable slide to accommodate different-size batteries. A Zener diode inside adapter regulates charging voltage. Adapter is about same size as a "D" cell and easily fits into battery charger shown in Fig. 3. A constant-voltage charger is the only kind that will handle these batteries properly. (The charger I used would be classified as a constant current type.) When the battery approaches full charge, the charging current falls practically to zero. The current must also be limited to a safe value. There are automatically regulated mercury battery chargers on the market. And an adapter the size of a D cell is available to charge hearing-aid batteries. It fits into the charger in lieu of a battery, and it uses a Zener diode to establish a constant-voltage type of charge. While mercury batteries can be recharged, chargers built to handle more than one type of battery would have to be a bit more elaborate, because of the variety of sizes and voltages. Danger of explosion is greater than with other types of cells. You can blow up a carbon-zinc cell, but only by charging it at a grossly excessive rate. Most such blow-ups of carbon-zinc cells are due to steam, and would be impossible at a 45-mA charging rate. In conclusion, dry cells can be easily recharged. If you can get about two hours or more out of a recharged cell, the job of removing, charging, and reinstalling the batteries is worthwhile. The electricity cost is small - it averages out to 3.4 cents per charge. Indeed, most of the power used is consumed in the dropping resistor. The better chargers use a transformer to deliver not more than about 3.5 volts to two cells connected in series. Overcharging is then impossible since the charge tapers off to nothing as the rising voltage of the cells approaches that of the supply. A resistor or capacitor across the rectifier to provide about a 10% reverse charging current could be desirable. Whether adding the a.c. component made the difference between the up-to-date results above and the rather inconclusive ones of the 1950's, I don't know. I suspect that leak-proof construction, now universal, may have been an important factor. Batteries don't dry out as quickly as they used to.
Posted September 11, 2023 |
|