Quartz Crystal Etching |
||
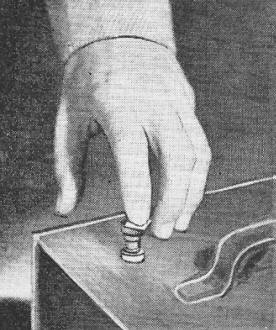
If you are going to write an article about etching crystals for radio frequency circuits, having a last name like Gene Brizendine's couldn't be much better. It just sounds like a mineral or a compound (benzidine, etc.). Amateur radio operators have for a long time adjusted the resonant frequency of their crystals by either grinding or chemical etching. Mr. Brizendine recommends etching as a more controllable method that does not reduce the quality of the crystal like improper grinding can do. Ammonium bifluoride is mentioned as the etchant. It is said to have cost about $1/pound in 1954, and sells on Amazon today for about $23/pound. According to the BLS Inflation Calculator, $1 in 1954 is the equivalent of about $9.60/pound in 2020, so the price has gone up considerably - not sure why. Of course it could be that the modern solution is more concentrated than that used by the author. Quartz Crystal Etching Non-standard or unusual frequencies can be obtained by etching standard or surplus crystals to desired range. Frequently, in experimental and research work, it is found that a crystal of an odd or unusual frequency is required. Placing an order and waiting for the plate to be specially processed is often expensive and time-consuming. The crystal etching process to be described solves this difficulty nicely, enabling anyone to adjust plates to the desired frequency quickly, and at very little expense. To explore these possibilities, the author successfully processed five surplus plates which were required to be spaced exactly 100 kilocycles apart in frequency. The crystals used were the type which fit the popular FT-243 holder and are suspended at each corner by "lands" or raised portions on the holder electrodes. Previous experience in changing the frequency of this type of crystal by grinding, had resulted in decreased activity and, in some cases, output had dropped to zero, in spite of all measures taken to restore activity. In almost all cases crystal operation was unsatisfactory. One cause for this appears to be that the crystals do not receive equal treatment over their surfaces. Another is that particles of both the grinding material and quartz may become embedded in the surface, hampering operation. The etching process removes material uniformly over all surfaces of a good-quality plate, including the edges, which are tedious to finish by other methods. When taken from the etching solution and rinsed, the plate is very clean. Items required for processing are pictured in Fig. 2. Fig. 1 - Checking the approximate frequency of plate with BC-221 frequency meter. Fig. 2 - Equipment needed to etch crystals. Ammonium bifluoride, a plastic ice cube tray, rubber gloves, and a frequency meter are needed to perform this operation. Before removing the crystals from their holders, they were plugged into a grid-dip meter, instead of the usual coil, to obtain an indication of their original activity. This reading, along with the frequency, was recorded for each plate. The empty holders were immediately closed again, protecting the electrodes from any dust or corroding effect of the air. Ammonium bifluoride, CP grade, was used for the processing. This material is inexpensive, costing about one dollar per pound, and may be obtained from a chemical supply house. The material should not contact any part of the body and although not highly toxic, the fumes should not be breathed. For these reasons, rubber gloves were used and the actual etching was done out-of-doors. The chemical attacks glass also, and for this reason, the usual container in which it is supplied is wax-lined. A saturated solution was prepared in a plastic dish, by dissolving all the ammonium bifluoride which would dissolve in one pint of water. The saturation point may be noted when particles no longer will dissolve and sink to the bottom of the container. Preparation of the solution may be speeded up somewhat by stirring and crushing the larger lumps. Etching is possible with a more dilute solution, however the rate will be proportionately slower. For a "test run", one plate was submerged in the solution, timing being done with an ordinary clock. This means was used to determine the speed of etching to be expected with the particular solution and crystal being processed. After exactly 60 minutes, the plate was removed and immediately rinsed in clean water. After drying, the frequency and activity were measured, again using the grid-dip meter and BC-221 frequency meter. The activity had actually improved and the frequency had increased from the original 4600 to 4603 kilocycles indicating an etching rate of 3 kilocycles per hour. With this figure established, the crystal was brought to the desired frequency easily, by appropriate timing. It was observed that different crystals of the same original frequency may exhibit widely differing etch rates. For this reason, each crystal was etched "privately," occupying one compartment of the plastic ice cube tray shown in Fig. 2. The etch-rate, the predicted date, and time the goal frequency would be reached, were penciled on strips of masking tape placed along the tray sides. The etching rate will be more constant by maintaining the solution at a recommended 60° centigrade, however, perfectly satisfactory results were obtained without this refinement. A rapid means of checking the approximate frequency of a plate was found, using the BC-221 frequency meter. As shown in Fig. 1, the plate is simply placed on the meter output binding post. The crystal is excited mechanically, by lightly tapping its center, while exploring with the main dial. At the general frequency of the crystal, loud plops are heard in the headphones. As the crystal neared the desired frequency, all measurements were made with the crystal operating in the equipment and holder to be used. Usual precautions in handling the finished blank should be observed to prevent oil from the hands reaching the surfaces. Should the electrodes appear soiled, they may be washed, using castile soap and a toothbrush. The writer prefers to dry the plate and electrodes in tissue, such as is used to clean optical lens, and then "pour" the plate into the holder, without actually touching either crystal or electrodes. The finished plate was installed between electrodes intended for operation near the desired frequency. This is necessary, since the height of the supporting corners varies with frequency. It is possible for the center of the plate to strike the electrode plate, if sufficient clearance is not provided by the "lands". On the other extreme, efficiency decreases if the corner supports are too high for a given frequency. With the crystal operating in the grid-dip meter, the position of the plate for best output was located, by making slight changes in its position between the electrodes, until the highest meter reading was obtained. The cover was then tightened and the activity again checked, to see that the same alignment was in effect. Crystals which failed to respond to the process were noted to have some visible flaw such as excessive air bubbles or chipped surfaces. Some plates ceased to oscillate because their composition was not uniform, developing a wood-grained appearance as the softer portions were etched away. Appreciation is expressed to Mr. R. B. Belser of the Georgia Institute of Technology staff for outlining the basic elements of this process. Reference Heising, Raymond A.: "Quartz Crystals for Electrical Circuits," D. Van Nostrand Company, Inc., New York.
Posted April 24, 2020 |
||