The Rechargeable Alkaline Battery
|
|
Nickel cadmium (NiCd or NiCad) and nickel metal hydride (NiMH) batteries are classified as secondary cells because they are rechargeable. Primary cells like carbon and standard alkaline are not rechargeable. Those were the most common types of batteries- at least for consumer products - up through the late 1980s. The now-common lithium ion (Li-Ion or LiIon) and lithium polymer (Li-Po or LiPo) are also secondary types. Devices designed to use either lithium chemistry are meant to work at integer multiples of the nominal 3.7 V cells potential. In the mid 1980s, when NiMH was gaining popularity as a high energy density rechargeable battery, a lot of people (including myself) began using them instead of standard alkaline batteries in cameras, radios, toys, etc. The problem was, though, that both NiCd and NiMH cells have a nominal voltage of just 1.2 V, which meant that the operation time of the device was often less than with a disposable alkaline or carbon battery. A lot of electronic devices designed for 1.5 V per cell barely function at 1.2 V. Rechargeable alkaline batteries, which still had a 1.5 V nominal voltage, were/are the compromise solution to that scenario. Energy density of an alkaline compared to a NiMH battery is typically much less, but they still provide better economy than using disposable batteries. New Battery Offers Economy and Long Operating Life By Samuel C. Milbourne The rechargeable alkaline is a relatively new type of battery. Similar in construction to the regular alkalines (but marked "rechargeable"), these batteries have a potential of 25 or more recharges. They require no added electrolyte or water: and they are available in the conventional 1.5-volt D, C, and AA sizes. The exclusive product of the Mallory Battery Co., the rechargeable alkaline should not be confused with nor can they be used to replace nickel-cadmium batteries. They can, however, be used for radios, cameras, toys, flashlights, portable TV receivers, record players, tape recorders, etc. Higher priced initially than carbon-zinc types, the rechargeable alkaline's cost, divided by the number of charges it can take, yields excellent overall economy. Rechargeable alkaline batteries are sold fully charged and have a shelf life of two years or more. Charging should be done at frequent intervals and always before they discharge below 1.2 volts. If the output is allowed to drop to 0.9 volt, these batteries may suffer irreparable damage. The AA, C, and D cells are sold two on a card and list for $2.00, $3.00, and $3.50, respectively, for the pair. (Fortunately, there is usually a substantial trade discount.) The applicable charger lists at $6.00. Specifications for the 1.5-volt battery types are listed in the Table. Mallory is also making available a 6-volt version of the alkaline rechargeable battery. It is roughly 6" high and weighs 3 1/2 pounds. It can furnish 2.5 amperes for 1 1/2 hours. The recharge capacity of this battery is 7 A-hr and a maximum recharge rate of 600 mA. It has an internal 10-ampere fuse; so, use a 5-ampere fuse externally. The rechargeable 6-volt battery is a natural for any type of portable or mobile application. Two in series can be used as a convenient bench supply for testing 12-volt solid-state mobile equipment. The charging time for any battery can be estimated from the recharge capacity of the battery in ampere-hours (A-hr) multiplied by the percentage for recharge losses. For example, the SA15AA battery's recharge capacity is 0.3 A-hr. If this battery is recharged at 13.5 mA for 33 hours, this would result in 0.445 A-hr - or 50 percent extra, which is an average amount.
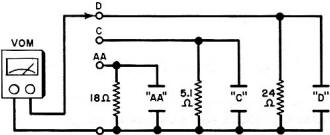
It is recommended that you make up some sort of chart to log all battery recharge times and dates. Make the charts small enough to be rubber-cemented or taped to the equipment in which the rechargeable alkaline batteries are used. Also, it is a good idea to run periodic voltage checks on the batteries in use. You can assemble a simple battery tester by following the circuit shown in Fig. 1. The indicating device to be used with this test circuit is a simple VOM. When should a battery be checked to determine if it is in need of a recharge? When the equipment in which it is used begins to malfunction - the receiver to distort, the record player to slow down, etc. - the batteries are ready for recharging. But you will obtain longer life from these batteries if you check them out and charge them more often. (Remember, never recharge a new battery.) One of the simplest battery chargers is an unregulated type, such as the Mallory Model BC-15 shown in Fig. 2. This unit will accommodate all three 1.5-volt cell sizes and charge them at the proper rates. The charger is very safe to handle. The step-down transformer is located in the line plug housing; so, no lethal or dangerous voltage levels appear in the charger itself. The stepped-down voltage is supplied to two separate charging circuits through separate diodes, current-limiting lamps and dropping resistors. There are three current controlled circuits available to each of the charging troughs. A clever device at the positive ends of the batteries makes contact with one of the three dropping resistors so that the proper charging current is applied to each of the three sizes. The current-limiting lamps are shown to the left of the batteries. One or both lamps lights up according to how the charger is loaded. Three levels of light are noticeable, one for each battery type, The charging levels are 27 m A, 80 mA, and 160 mA for the AA, C, and D cells, respectively.
Posted February 29, 2024 |
|


 Charging rates for rechargeable alkaline batteries
can be increased, thus decreasing the charging time required, if a voltage-limiting
charger circuit is used. This would remove the battery electrically from the charging
circuit when the desired voltage level is attained. However, if the previously stated
rates and charging times (see Table) are used as a guide, or the maker's relatively
simple charger is used, nothing more is needed except patience.
Charging rates for rechargeable alkaline batteries
can be increased, thus decreasing the charging time required, if a voltage-limiting
charger circuit is used. This would remove the battery electrically from the charging
circuit when the desired voltage level is attained. However, if the previously stated
rates and charging times (see Table) are used as a guide, or the maker's relatively
simple charger is used, nothing more is needed except patience.