Inside the Dry Cell
|
|
Dry cell chemistry has come a long way since this article appeared in the April 1959 edition of Popular Electronics magazine. Yes, you can still buy a basic carbon battery, but much superior cells are available now that perform over much wider temperature ranges, have nearly flat discharge curves throughout their rated range, and offer standard chemistries with voltages other than 1.5 volts per cell (nominal). For example, nickel metal hydride (NiMH) cells exhibit 1.2 volts nominally, and lithium polymer (LiPo*) cells have a nominal voltage of 3.7 volts. As a kid in the 1960s and 70s, I spent a lot of time struggling to make Eveready flashlight "D" cells from my father's flashlight power the glow plugs on my model airplane engines. They were typically so weak that even slight flooding from glow fuel on the element that the current could not get nichrome elements hot enough. Nowadays, a voltage regulated glow plug driver can overcome any amount of fuel on the heating element. I have seen photos of glow plugs immersed in glow fuel and still glowing brightly while submerged. * BTW, did you know there was an 8th century Chinese poet named Li Po; he showed a real potential for versification. Inside the Dry Cell By Saunder Harris WINXL
What Goes On In Fig. 1, a typical dry cell is shown in cross section. Its zinc outer case serves as the negative electrode of the cell. The positive electrode is formed by a cylindrical carbon rod in the center of the cell. Separating the two electrodes is a pasty substance composed of an electrolyte and a depolarizing mix. Due to chemical action between the electrolyte (ammonium chloride) and the zinc case, electrons pile up on the sides of the zinc container and bubbles of hydrogen gas travel through the electrolyte and cling to the sides of the carbon rod. As the bubbles pile up, they tend to choke off the action of the cell. Here is where the depolarizing mix goes to work. Since it is composed of manganese dioxide, which has a high content of oxygen, it mixes its oxygen with the hydrogen bubbles and water is formed. This gets rid of the unwanted hydrogen and also keeps the electrolyte from drying up.
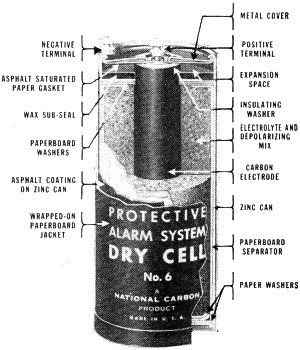 Fig. 1 - Cross-section view of a typical dry cell.
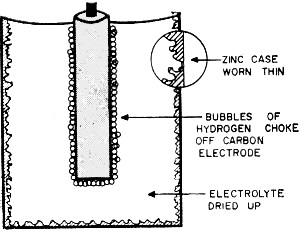
Fig. 2 - Internal condition of dry cell at exhaustion.
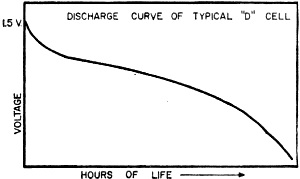
Fig. 3 - Gradual battery volt-age drop with time and use. Now let's see what happens when the battery is exhausted. The zinc walls of the cell get thinner and thinner, and the electrolyte - instead of being in paste form - dries out into powder. The depolarizing mix stops doing its job and the hydrogen bubbles around the positive carbon element just about stop all flow of electrons. Figure 2 will give you some idea of the condition of the cell at this point. This dry cell has had it. Life of a Battery Knowing what goes on inside the cell, it is now time to answer the question most important to you as a battery user. How long will your batteries last? According to the National Bureau of Standards, in 1910, under standard testing procedures, a size "D" cell would give 260 minutes of service under intermittent use. In 1951, the testing of 12 to 15 brands of the same size "D" cell showed an average service life of over 800 minutes, with some cells giving 1000 to 1100 minutes of service. Today's batteries will do even better than that with proper care. The five factors that determine the life of a dry cell battery are: (1) Initial current drain (2) Hours of use per day (3) End point voltage (4) Temperature (5) Storage period prior to use It is impossible to say that any battery has an exact number of hours of service life. If a battery is operated under conditions which draw a large current from it in a short period of time, the depolarizing mix cannot do its job properly and the voltage will drop off very rapidly. This is the situation we have already discussed. On the other hand, if the battery is used too slowly, its normal aging will cause the output to be reduced. The shelf life of a battery can range from a few months to as long as two years, depending upon the type of battery and the conditions of storage. While in use, your battery should periodically be given "time off" to allow the depolarizing mix to work and remove the hydrogen and other waste products developed in the cell. This point is emphasized because it is so important in proper battery care. It will pay you to have two sets of batteries for frequently operated devices. By switching from set to set, you will increase the operating life of both sets. The end point voltage is of interest mainly to the designer of the battery-operated device rather than the user, and so we will only touch on it here. The end point voltage is the voltage below which the battery can no longer operate the device in question. If it takes one volt per cell to operate a radio receiver, the unit will not operate when the battery voltage goes to 0.9 volt per cell. The best designs make the end point voltage as low as possible to allow the maximum to be gotten from a battery as its voltage drops off with time and use. Figure 3 will give you some idea of the manner in which battery voltage drops off with time. The Temperature Story Dry cell batteries designed for normal use operate best at room temperature, about 70° F. When batteries are exposed to continual high temperatures, they will break down in a much shorter time due to increased chemical action and a drying up of the electrolyte. Low temperatures, however, are a different story. From the standpoint of storage, a battery loves cold weather. For example, as you may know, batteries which were frozen in the Arctic ice on polar expeditions were thawed out years later by other explorers and found quite usable. Cold will slow up the chemical action within a dry cell battery and in some cases make it inoperative; but when it is brought back to room temperature, the battery will return to normal operation none the worse for the chilling. If you are going to store a dry cell battery for any length of time, store it in a cool spot. A temperature of about 45° F is ideal, and a shelf in the refrigerator is an excellent storage spot (if you can get away with it). According to tests made by the National Carbon Company's Battery Engineering Department, a battery which has been stored in this manner for nine months will give as much useful service as will a battery stored at room temperature for three months. Just remember to take the battery out of the cold about six hours prior to using it, and allow it to get back to room temperature for normal operation. "Batteries" and "Cells" Before we complete this discussion of basic dry cells, there are a few things which deserve clarification. You will note that we have used the term "cell" and "battery." A cell is one unit consisting of positive and negative electrodes separated by an electrolyte. A battery is two or more cells, connected in series to add their voltage, and packaged in one container. Actually, the "battery" you put in your flashlight is a cell, while the B+ unit you use in your portable radio is a true battery because it consists of more than one cell. You should also be aware that every cell, no matter what its electrode materials, develops about 1 1/2 volts. The physical size of the cell determines the amount of current that can be drawn from it. There is, of course, much about dry cell batteries that we haven't discussed. There are also many new types of batteries such as the mercury battery, the solar battery and the new rechargeable dry cells, all of which are now important means of developing portable power. These we will cover in a future issue. However, the dry cells that we have considered are still the workhorses of the battery world and, with a little care, will pay you many dividend hours of extra, dependable service.
Posted January 6, 2022 |
|

 A dry cell is a "package of electricity" which
produces electrical energy by chemical means. A quick look inside a portable radio,
a flashlight or a hearing aid will reveal one or more of these compact power sources
ready to deliver the juice at the flip of a switch. How does the dry
cell produce electricity? Without going into chemical reaction formulas, let's take
a look at what goes on inside a dry cell.
A dry cell is a "package of electricity" which
produces electrical energy by chemical means. A quick look inside a portable radio,
a flashlight or a hearing aid will reveal one or more of these compact power sources
ready to deliver the juice at the flip of a switch. How does the dry
cell produce electricity? Without going into chemical reaction formulas, let's take
a look at what goes on inside a dry cell.