| 1 |
2.778 * 10-3 |
10 |
2.998 * 1010 |
| 360 |
1 |
3600 |
1.079 * 1013 |
| 0.1 |
2.778 * 10-4 |
1 |
2.998 * 109 |
| 3.336 * 10-11 |
9.266 * 10-14 |
3.336 * 10-10 |
1 |
Note: The prefix "ab" is used to indicate
an electromagnetic unit in the centimeter-gram-second system.
The prefix "stat"
is used to indicate an electrical unit in the electrostatic centimeter-gram-second
system of units.
Standard unit = Coulomb (C) |

Electric charge is a fundamental property of matter that describes the intrinsic
electrical property of particles such as electrons and protons. It is one of the
key concepts in physics and plays a fundamental role in understanding the behavior
of matter and the interactions between particles. Here are some key points about
electric charge:
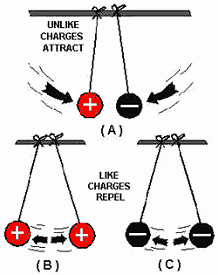 Types of Electric Charge: Types of Electric Charge:
- There are two types of electric charge: positive and negative.
- Protons are positively charged, while electrons are negatively charged.
- Positive charges repel each other, and negative charges repel each other, but
positive and negative charges attract each other.
Conservation of Electric Charge:
The principle of conservation of electric charge states that the total electric
charge in a closed system remains constant. In other words, electric charge cannot
be created or destroyed, only transferred from one object to another.
Quantization of Electric Charge:
- Electric charge is quantized, meaning it comes in discrete, indivisible units.
- The elementary charge (e) is the charge of a single proton or electron and is
approximately equal to 1.602 x 10^-19 coulombs (C).
Charge Interactions:
- Coulomb's Law describes the force of attraction or repulsion between two charged
objects. It states that the force is directly proportional to the product of the
magnitudes of the charges and inversely proportional to the square of the distance
between them.
- The direction of the force depends on the types of charges involved (attractive
or repulsive).
Charge in Everyday Life:
- Electric charge is responsible for many everyday phenomena, including the operation
of electronic devices, the flow of current in electrical circuits, and the behavior
of magnets.
- It is also responsible for static electricity, where objects can become charged
due to friction and can either attract or repel each other.
Units of Electric Charge:
- The SI unit of electric charge is the coulomb (C). One coulomb is equal to the
charge of approximately 6.242 x 10^18 protons or electrons.
- Smaller units, such as the milliampere-hour (mAh) or microcoulomb (µC), are
often used in practical applications.
Posted September 11, 2023
(updated from original
post on 4/23/2001)
|

 Types of Electric Charge:
Types of Electric Charge: